Identification of OmpR-family response regulators interacting with thioredoxin in the Cyanobacterium Synechocystis sp. PCC 6803
- PMID: 25774906
- PMCID: PMC4361706
- DOI: 10.1371/journal.pone.0119107
Identification of OmpR-family response regulators interacting with thioredoxin in the Cyanobacterium Synechocystis sp. PCC 6803
Erratum in
-
Correction: identification of OmpR-family response regulators interacting with thioredoxin in the cyanobacterium Synechocystis sp. PCC 6803.PLoS One. 2015 Apr 13;10(4):e0124571. doi: 10.1371/journal.pone.0124571. eCollection 2015. PLoS One. 2015. PMID: 25875789 Free PMC article.
Abstract
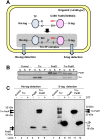
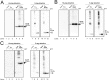
The redox state of the photosynthetic electron transport chain is known to act as a signal to regulate the transcription of key genes involved in the acclimation responses to environmental changes. We hypothesized that the protein thioredoxin (Trx) acts as a mediator connecting the redox state of the photosynthetic electron transport chain and transcriptional regulation, and established a screening system to identify transcription factors (TFs) that interact with Trx. His-tagged TFs and S-tagged mutated form of Trx, TrxMC35S, whose active site cysteine 35 was substituted with serine to trap the target interacting protein, were co-expressed in E. coli cells and Trx-TF complexes were detected by immuno-blotting analysis. We examined the interaction between Trx and ten OmpR family TFs encoded in the chromosome of the cyanobacterium Synechocystis sp. PCC 6803 (S.6803). Although there is a highly conserved cysteine residue in the receiver domain of all OmpR family TFs, only three, RpaA (Slr0115), RpaB (Slr0947) and ManR (Slr1837), were identified as putative Trx targets [corrected].The recombinant forms of wild-type TrxM, RpaA, RpaB and ManR proteins from S.6803 were purified following over-expression in E. coli and their interaction was further assessed by monitoring changes in the number of cysteine residues with free thiol groups. An increase in the number of free thiols was observed after incubation of the oxidized TFs with Trx, indicating the reduction of cysteine residues as a consequence of interaction with Trx. Our results suggest, for the first time, the possible regulation of OmpR family TFs through the supply of reducing equivalents from Trx, as well as through the phospho-transfer from its cognate sensor histidine kinase.
Conflict of interest statement
Figures



Similar articles
-
Thiol redox switches regulate the oligomeric state of cyanobacterial Rre1, RpaA and RpaB response regulators.FEBS Lett. 2022 Jun;596(12):1533-1543. doi: 10.1002/1873-3468.14340. Epub 2022 Apr 11. FEBS Lett. 2022. PMID: 35353903 Free PMC article.
-
The PedR transcriptional regulator interacts with thioredoxin to connect photosynthesis with gene expression in cyanobacteria.Biochem J. 2010 Oct 1;431(1):135-40. doi: 10.1042/BJ20100789. Biochem J. 2010. PMID: 20662766
-
Photosynthetic regulation of the cyanobacterium Synechocystis sp. PCC 6803 thioredoxin system and functional analysis of TrxB (Trx x) and TrxQ (Trx y) thioredoxins.Mol Plant. 2009 Mar;2(2):270-83. doi: 10.1093/mp/ssn070. Epub 2008 Nov 17. Mol Plant. 2009. PMID: 19825613
-
Thioredoxin and its related molecules: update 2005.Antioxid Redox Signal. 2005 May-Jun;7(5-6):823-8. doi: 10.1089/ars.2005.7.823. Antioxid Redox Signal. 2005. PMID: 15890030 Review.
-
The OmpR-family of proteins: insight into the tertiary structure and functions of two-component regulator proteins.J Biochem. 2001 Mar;129(3):343-50. doi: 10.1093/oxfordjournals.jbchem.a002863. J Biochem. 2001. PMID: 11226872 Review.
Cited by
-
Nrf1 acts as a highly-conserved determinon for maintaining robust redox homeostasis in the eco-evo-devo process of life histories.Cell Stress. 2025 Jul 7;9:65-142. doi: 10.15698/cst2025.07.306. eCollection 2025. Cell Stress. 2025. PMID: 40703332 Free PMC article. Review.
-
Thiol redox switches regulate the oligomeric state of cyanobacterial Rre1, RpaA and RpaB response regulators.FEBS Lett. 2022 Jun;596(12):1533-1543. doi: 10.1002/1873-3468.14340. Epub 2022 Apr 11. FEBS Lett. 2022. PMID: 35353903 Free PMC article.
-
Biocomputational Analyses and Experimental Validation Identify the Regulon Controlled by the Redox-Responsive Transcription Factor RpaB.iScience. 2019 May 31;15:316-331. doi: 10.1016/j.isci.2019.04.033. Epub 2019 Apr 28. iScience. 2019. PMID: 31103851 Free PMC article.
-
Deciphering the Role of Multiple Thioredoxin Fold Proteins of Leptospirillum sp. in Oxidative Stress Tolerance.Int J Mol Sci. 2020 Mar 10;21(5):1880. doi: 10.3390/ijms21051880. Int J Mol Sci. 2020. PMID: 32164170 Free PMC article.
-
Epinephrine Affects Ribosomes, Cell Division, and Catabolic Processes in Micrococcus luteus Skin Strain C01: Revelation of the Conditionally Extensive Hormone Effect Using Orbitrap Mass Spectrometry and Proteomic Analysis.Microorganisms. 2023 Aug 29;11(9):2181. doi: 10.3390/microorganisms11092181. Microorganisms. 2023. PMID: 37764026 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

