IL-13 induces YY1 through the AKT pathway in lung fibroblasts
- PMID: 25775215
- PMCID: PMC4361578
- DOI: 10.1371/journal.pone.0119039
IL-13 induces YY1 through the AKT pathway in lung fibroblasts
Abstract
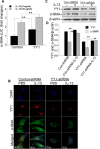
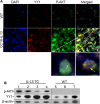
A key feature of lung fibrosis is the accumulation of myofibroblasts. Interleukin 13 (IL-13) is a pro-fibrotic mediator that directly and indirectly influences the activation of myofibroblasts. Transforming growth factor beta (TGF-β) promotes the differentiation of fibroblasts into myofibroblasts, and can be regulated by IL-13. However, IL-13's downstream signaling pathways are not completely understood. We previously reported that the transcription factor Yin Yang 1 (YY1) is upregulated in fibroblasts treated with TGF-β and in the lungs of mice and patients with pulmonary fibrosis. Moreover, YY1 directly regulates collagen and alpha smooth muscle actin (α-SMA) expression in fibroblasts. However, it is not known if IL-13 regulates fibroblast activation through YY1 expression. We hypothesize that IL-13 up-regulates YY1 expression through regulation of AKT activation, leading to fibroblast activation. In this study we found that YY1 was upregulated by IL-13 in lung fibroblasts in a dose- and time-dependent manner, resulting in increased α-SMA. Conversely, knockdown of YY1 blocked IL-13-induced α-SMA expression in fibroblasts. Furthermore, AKT phosphorylation was increased in fibroblasts treated with IL-13, and AKT overexpression upregulated YY1, whereas blockade of AKT phosphorylation suppressed the induction of YY1 by IL-13 in vitro. In vivo YY1 was upregulated in fibrotic lungs from CC10-IL-13 transgenic mice compared to that from wild-type littermates, which was associated with increased AKT phosphorylation. Taken together, these findings demonstrate that IL-13 is a potent stimulator and activator of fibroblasts, at least in part, through AKT-mediated YY1 activation.
Conflict of interest statement
Figures



Similar articles
-
Yin yang 1 is a novel regulator of pulmonary fibrosis.Am J Respir Crit Care Med. 2011 Jun 15;183(12):1689-97. doi: 10.1164/rccm.201002-0232OC. Epub 2010 Dec 17. Am J Respir Crit Care Med. 2011. PMID: 21169469 Free PMC article.
-
YY1 mediates TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial cells.Respir Res. 2019 Nov 8;20(1):249. doi: 10.1186/s12931-019-1223-7. Respir Res. 2019. PMID: 31703732 Free PMC article.
-
The inhibitory effect of ginsan on TGF-β mediated fibrotic process.J Cell Physiol. 2011 May;226(5):1241-7. doi: 10.1002/jcp.22452. J Cell Physiol. 2011. PMID: 20945375
-
Plasminogen activator inhibitor-1 promotes the proliferation and inhibits the apoptosis of pulmonary fibroblasts by Ca(2+) signaling.Thromb Res. 2013 Jan;131(1):64-71. doi: 10.1016/j.thromres.2012.09.003. Epub 2012 Sep 25. Thromb Res. 2013. PMID: 23021499
-
CCN5 overexpression inhibits profibrotic phenotypes via the PI3K/Akt signaling pathway in lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis and in an in vivo model of lung fibrosis.Int J Mol Med. 2014 Feb;33(2):478-86. doi: 10.3892/ijmm.2013.1565. Epub 2013 Nov 25. Int J Mol Med. 2014. PMID: 24276150
Cited by
-
Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice.Mol Ther. 2019 Mar 6;27(3):571-583. doi: 10.1016/j.ymthe.2019.01.008. Epub 2019 Jan 18. Mol Ther. 2019. PMID: 30711446 Free PMC article.
-
Exogenous miR-29a Attenuates Muscle Atrophy and Kidney Fibrosis in Unilateral Ureteral Obstruction Mice.Hum Gene Ther. 2020 Mar;31(5-6):367-375. doi: 10.1089/hum.2019.287. Epub 2020 Feb 21. Hum Gene Ther. 2020. PMID: 31950871 Free PMC article.
-
The Selective Angiotensin II Type 2 Receptor Agonist, Compound 21, Attenuates the Progression of Lung Fibrosis and Pulmonary Hypertension in an Experimental Model of Bleomycin-Induced Lung Injury.Front Physiol. 2018 Mar 27;9:180. doi: 10.3389/fphys.2018.00180. eCollection 2018. Front Physiol. 2018. PMID: 29636695 Free PMC article.
-
The Effect of Nintedanib on T-Cell Activation, Subsets and Functions.Drug Des Devel Ther. 2021 Mar 8;15:997-1011. doi: 10.2147/DDDT.S288369. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33727792 Free PMC article.
-
Interleukin-13 and age-related macular degeneration.Int J Ophthalmol. 2017 Apr 18;10(4):535-540. doi: 10.18240/ijo.2017.04.06. eCollection 2017. Int J Ophthalmol. 2017. PMID: 28503424 Free PMC article.
References
-
- Gharaee-Kermani M, Hu B, Thannickal VJ, Phan SH, Gyetko MR (2007) Current and emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs 12: 627–646. - PubMed
-
- Roy SG, Nozaki Y, Phan SH (2001) Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol 33: 723–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

