The 1.7 Å X-ray crystal structure of the porcine factor VIII C2 domain and binding analysis to anti-human C2 domain antibodies and phospholipid surfaces
- PMID: 25775247
- PMCID: PMC4361576
- DOI: 10.1371/journal.pone.0122447
The 1.7 Å X-ray crystal structure of the porcine factor VIII C2 domain and binding analysis to anti-human C2 domain antibodies and phospholipid surfaces
Abstract
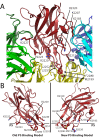
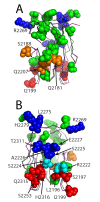
The factor VIII C2 domain is essential for binding to activated platelet surfaces as well as the cofactor activity of factor VIII in blood coagulation. Inhibitory antibodies against the C2 domain commonly develop following factor VIII replacement therapy for hemophilia A patients, or they may spontaneously arise in cases of acquired hemophilia. Porcine factor VIII is an effective therapeutic for hemophilia patients with inhibitor due to its low cross-reactivity; however, the molecular basis for this behavior is poorly understood. In this study, the X-ray crystal structure of the porcine factor VIII C2 domain was determined, and superposition of the human and porcine C2 domains demonstrates that most surface-exposed differences cluster on the face harboring the "non-classical" antibody epitopes. Furthermore, antibody-binding results illustrate that the "classical" 3E6 antibody can bind both the human and porcine C2 domains, although the inhibitory titer to human factor VIII is 41 Bethesda Units (BU)/mg IgG versus 0.8 BU/mg IgG to porcine factor VIII, while the non-classical G99 antibody does not bind to the porcine C2 domain nor inhibit porcine factor VIII activity. Further structural analysis of differences between the electrostatic surface potentials suggest that the C2 domain binds to the negatively charged phospholipid surfaces of activated platelets primarily through the 3E6 epitope region. In contrast, the G99 face, which contains residue 2227, should be distal to the membrane surface. Phospholipid binding assays indicate that both porcine and human factor VIII C2 domains bind with comparable affinities, and the human K2227A and K2227E mutants bind to phospholipid surfaces with similar affinities as well. Lastly, the G99 IgG bound to PS-immobilized factor VIII C2 domain with an apparent dissociation constant of 15.5 nM, whereas 3E6 antibody binding to PS-bound C2 domain was not observed.
Conflict of interest statement
Figures




References
-
- Hoots WK. The future of plasma-derived clotting factor concentrates. Haemophilia: the official journal of the World Federation of Hemophilia. 2001;7 Suppl 1:4–9. - PubMed
-
- Mauser-Bunschoten EP, van der Bom JG, Bongers M, Twijnstra M, Roosendaal G, Fischer K, et al. Purity of factor VIII product and incidence of inhibitors in previously untreated patients with haemophilia A. Haemophilia: the official journal of the World Federation of Hemophilia. 2001;7(4):364–8. - PubMed
-
- Scharrer I, Bray GL, Neutzling O. Incidence of inhibitors in haemophilia A patients—a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Haemophilia: the official journal of the World Federation of Hemophilia. 1999;5(3):145–54. - PubMed
-
- Bray GL, Gomperts ED, Courter S, Gruppo R, Gordon EM, Manco-Johnson M, et al. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood. 1994;83(9):2428–35. - PubMed
-
- Kreuz W, Ettingshausen CE, Zyschka A, Oldenburg J, Saguer IM, Ehrenforth S, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Seminars in thrombosis and hemostasis. 2002;28(3):285–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

