Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States
- PMID: 25775312
- PMCID: PMC4435698
- DOI: 10.7326/M14-1336
Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States
Abstract
Background: Sofosbuvir and ledipasvir, which have recently been approved for treatment of chronic hepatitis C virus (HCV) infection, are more efficacious and safer than the old standard of care (oSOC) but are substantially more expensive. Whether and in which patients their improved efficacy justifies their increased cost is unclear.
Objective: To evaluate the cost-effectiveness and budget impact of sofosbuvir and ledipasvir.
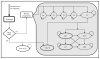
Design: Microsimulation model of the natural history of HCV infection.
Data sources: Published literature.
Target population: Treatment-naive and treatment-experienced HCV population defined on the basis of HCV genotype, age, and fibrosis distribution in the United States.
Time horizon: Lifetime.
Perspective: Third-party payer.
Intervention: Simulation of sofosbuvir-ledipasvir compared with the oSOC (interferon-based therapies).
Outcome measures: Quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and 5-year spending on antiviral drugs.
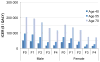
Results of base-case analysis: Sofosbuvir-based therapies added 0.56 QALY relative to the oSOC at an ICER of $55 400 per additional QALY. The ICERs ranged from $9700 to $284 300 per QALY depending on the patient's status with respect to treatment history, HCV genotype, and presence of cirrhosis. At a willingness-to-pay threshold of $100 000 per QALY, sofosbuvir-based therapies were cost-effective in 83% of treatment-naive and 81% of treatment-experienced patients. Compared with the oSOC, treating eligible HCV-infected persons in the United States with the new drugs would cost an additional $65 billion in the next 5 years, whereas the resulting cost offsets would be $16 billion.
Results of sensitivity analysis: Results were sensitive to drug price, drug efficacy, and quality of life after successful treatment.
Limitation: Data on real-world effectiveness of new antivirals are lacking.
Conclusion: Treatment of HCV is cost-effective in most patients, but additional resources and value-based patient prioritization are needed to manage patients with HCV.
Primary funding source: National Institutes of Health.
Conflict of interest statement
Figures
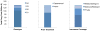


References
-
- Rosen HR. Chronic Hepatitis C Infection. New England Journal of Medicine. 2011;364(25):2429–38. - PubMed
-
- FDA Press Release. [Accessed March 18, 2014];FDA approves Sovaldi for chronic hepatitis C. 2013 Dec 3; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm377888.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
