Magnitude of the benefit of progression-free survival as a potential surrogate marker in phase 3 trials assessing targeted agents in molecularly selected patients with advanced non-small cell lung cancer: systematic review
- PMID: 25775395
- PMCID: PMC4361736
- DOI: 10.1371/journal.pone.0121211
Magnitude of the benefit of progression-free survival as a potential surrogate marker in phase 3 trials assessing targeted agents in molecularly selected patients with advanced non-small cell lung cancer: systematic review
Abstract
Background: In evaluation of the clinical benefit of a new targeted agent in a phase 3 trial enrolling molecularly selected patients with advanced non-small cell lung cancer (NSCLC), overall survival (OS) as an endpoint seems to be of limited use because of a high level of treatment crossover for ethical reasons. A more efficient and useful indicator for assessing efficacy is needed.
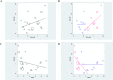
Methods and findings: We identified 18 phase 3 trials in the literature investigating EGFR-tyrosine kinase inhibitor (TKIs) or ALK-TKIs, now approved for use to treat NSCLC, compared with standard cytotoxic chemotherapy (eight trials were performed in molecularly selected patients and ten using an "all-comer" design). Receiver operating characteristic analysis was used to identify the best threshold by which to divide the groups. Although trials enrolling molecularly selected patients and all-comer trials had similar OS-hazard ratios (OS-HRs) (0.99 vs. 1.04), the former exhibited greater progression-free survival-hazard ratios (PFS-HR) (mean, 0.40 vs. 1.01; P<0.01). A PFS-HR of 0.60 successfully distinguished between the two types of trials (sensitivity 100%, specificity 100%). The odds ratio for overall response was higher in trials with molecularly selected patients than in all-comer trials (mean: 6.10 vs. 1.64; P<0.01). An odds ratio of 3.40 for response afforded a sensitivity of 88% and a specificity of 90%.
Conclusion: The notably enhanced PFS benefit was quite specific to trials with molecularly selected patients. A PFS-HR cutoff of ∼0.6 may help detect clinical benefit of molecular targeted agents in which OS is of limited use, although desired threshold might differ in an individual trial.
Conflict of interest statement
Figures



References
-
- Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M: Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22:3852–3859, 2004. - PubMed
-
- Hotta K, Matsuo K: Long-standing debate on cisplatin- versus carboplatin-based chemotherapy in the treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2:96, 2007. - PubMed
-
- Fujiwara Y, Matsuo K, Suzuki T, Kiura K, Tabata M, et al.: Recent improvement in the survival of patients with advanced non-small-cell lung cancer enrolled in phase III trials of first-line systemic chemotherapy. Cancer 109:939–948, 2007. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–2103, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

