Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti
- PMID: 25775608
- PMCID: PMC4386333
- DOI: 10.1073/pnas.1502370112
Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti
Abstract
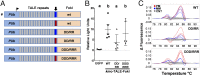
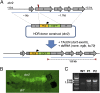
Conventional control strategies for mosquito-borne pathogens such as malaria and dengue are now being complemented by the development of transgenic mosquito strains reprogrammed to generate beneficial phenotypes such as conditional sterility or pathogen resistance. The widespread success of site-specific nucleases such as transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 in model organisms also suggests that reprogrammable gene drive systems based on these nucleases may be capable of spreading such beneficial phenotypes in wild mosquito populations. Using the mosquito Aedes aegypti, we determined that mutations in the FokI domain used in TALENs to generate obligate heterodimeric complexes substantially and significantly reduce gene editing rates. We found that CRISPR/Cas9-based editing in the mosquito Ae. aegypti is also highly variable, with the majority of guide RNAs unable to generate detectable editing. By first evaluating candidate guide RNAs using a transient embryo assay, we were able to rapidly identify highly effective guide RNAs; focusing germ line-based experiments only on this cohort resulted in consistently high editing rates of 24-90%. Microinjection of double-stranded RNAs targeting ku70 or lig4, both essential components of the end-joining response, increased recombination-based repair in early embryos as determined by plasmid-based reporters. RNAi-based suppression of Ku70 concurrent with embryonic microinjection of site-specific nucleases yielded consistent gene insertion frequencies of 2-3%, similar to traditional transposon- or ΦC31-based integration methods but without the requirement for an initial docking step. These studies should greatly accelerate investigations into mosquito biology, streamline development of transgenic strains for field releases, and simplify the evaluation of novel Cas9-based gene drive systems.
Keywords: Aedes; CRISPR; TALEN; recombination; transgenic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Harris AF, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29(11):1034–1037. - PubMed
-
- Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–457. - PubMed
-
- Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59:205–224. - PubMed
-
- Oye KA, et al. Biotechnology. Regulating gene drives. Science. 2014;345(6197):626–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

