4-Phenylbutyric Acid (4-PBA) and Lithium Cooperatively Attenuate Cell Death during Oxygen-Glucose Deprivation (OGD) and Reoxygenation
- PMID: 25776137
- PMCID: PMC11486266
- DOI: 10.1007/s10571-015-0179-5
4-Phenylbutyric Acid (4-PBA) and Lithium Cooperatively Attenuate Cell Death during Oxygen-Glucose Deprivation (OGD) and Reoxygenation
Abstract
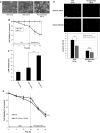
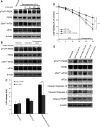
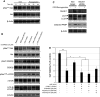
Hypoxia is an important cause of brain injury in ischemic stroke. It is known that endoplasmic reticulum (ER) stress is an important determinant of cell survival or death during hypoxia. However, the signaling pathways and molecular mechanisms involved remain to be studied in more detail. To investigate whether inhibition of ER stress promotes neuroprotection pathways, we applied an in vitro oxygen-glucose deprivation (OGD) followed by reoxygenation model of human SK-N-MC neuronal cell cultures in this study. Our results showed that neuronal cell death was induced in this model during the OGD reoxygenation by the sustained ER stress, but not during OGD phase. However, treatment of the cultures with lithium with the OGD reoxygenation insult did not result in neuroprotection, whereas concomitant treatment of chemical chaperon 4-phenylbutyric acid (4-PBA) provides protective effects in ER stress-exposed cells. Moreover, 4-PBA rescued ER stress-suppressed Akt protein biosynthesis, which works cooperatively with lithium in the activation of Akt downstream signaling by inhibition of autophagy-induced cell death. Taken together, our finding provides a possible mechanism by which 4-PBA and lithium contribute to mediate neuroprotection cooperatively. This result may potentially be a useful therapeutic strategy for ischemic stroke.
Figures

References
-
- Bando Y, Ogawa S, Yamauchi A, Kuwabara K, Ozawa K, Hori O, Yanagi H, Tamatani M, Tohyama M (2000) 150-kDa oxygen-regulated protein (ORP150) functions as a novel molecular chaperone in MDCK cells. Am J Physiol Cell Physiol 278(6):C1172–1182 - PubMed
-
- Box AH, Kim SM, Demetrick DJ (2010) AKT loss in human epithelial cells treated with severe hypoxia. Biochim Biophys Acta 1803(8):951–959. doi:10.1016/j.bbamcr.2010.03.011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

