Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells
- PMID: 25776754
- PMCID: PMC4432743
- DOI: 10.1128/IAI.00135-15
Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells
Abstract
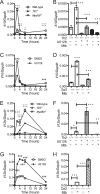
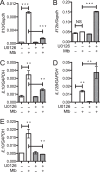
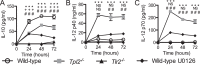
Mycobacterium tuberculosis survives within macrophages and employs immune evasion mechanisms to persist in the host. Protective T helper type 1 (Th1) responses are induced, and the immune response in most individuals is sufficient to restrict M. tuberculosis to latent infection, but most infections are not completely resolved. As T cells and macrophages respond, a balance is established between protective Th1-associated and other proinflammatory cytokines, such as interleukin-12 (IL-12), interferon gamma (IFN-γ), and tumor necrosis factor alpha, and anti-inflammatory cytokines, such as IL-10. The mechanisms by which M. tuberculosis modulates host responses to promote its survival remain unclear. In these studies, we demonstrate that M. tuberculosis induction of IL-10, suppression of IL-12, and inhibition of class II major histocompatibility complex (MHC-II) molecules in infected macrophages are all driven by Toll-like receptor 2 (TLR2)-dependent activation of the extracellular signal-regulated kinases (ERK). Elimination of ERK signaling downstream of TLR2 by pharmacologic inhibition with U0126 or genetic deletion of Tpl2 blocks IL-10 secretion and enhances IL-12 p70 secretion. We demonstrate that M. tuberculosis regulation of these pathways in macrophages affects T cell responses to infected macrophages. Thus, genetic blockade of the ERK pathway in Tpl2(-/-) macrophages enhances Th1 polarization and IFN-γ production by antigen-specific CD4(+) T cells responding to M. tuberculosis infection. These data indicate that M. tuberculosis and its potent TLR2 ligands activate ERK signaling in macrophages to promote anti-inflammatory macrophage responses and blunt Th1 responses against the pathogen.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Yahagi A, Umemura M, Tamura T, Kariyone A, Begum MD, Kawakami K, Okamoto Y, Hamada S, Oshiro K, Kohama H, Arakawa T, Ohara N, Takatsu K, Matsuzaki G. 2010. Suppressed induction of mycobacterial antigen-specific Th1-type CD4+ T cells in the lung after pulmonary mycobacterial infection. Int Immunol 22:307–318. doi:10.1093/intimm/dxq010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

