Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells
- PMID: 25778531
- PMCID: PMC4408295
- DOI: 10.1182/blood-2014-11-611707
Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells
Abstract
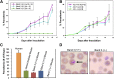
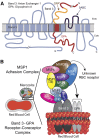
Plasmodium falciparum invasion of human red blood cells (RBCs) is an intricate process requiring a number of distinct ligand-receptor interactions at the merozoite-erythrocyte interface. Merozoite surface protein 1 (MSP1), a highly abundant ligand coating the merozoite surface in all species of malaria parasites, is essential for RBC invasion and considered a leading candidate for inclusion in a multiple-subunit vaccine against malaria. Our previous studies identified an interaction between the carboxyl-terminus of MSP1 and RBC band 3. Here, by employing phage display technology, we report a novel interaction between the amino-terminus of MSP1 and RBC glycophorin A (GPA). Mapping of the binding domains established a direct interaction between malaria MSP1 and human GPA within a region of MSP1 known to potently inhibit P falciparum invasion of human RBCs. Furthermore, a genetically modified mouse model lacking the GPA- band 3 complex in RBCs is completely resistant to malaria infection in vivo. These findings suggest an essential role of the MSP1-GPA-band 3 complex during the initial adhesion phase of malaria parasite invasion of RBCs.
© 2015 by The American Society of Hematology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

