Med1 regulates meiotic progression during spermatogenesis in mice
- PMID: 25778538
- PMCID: PMC4417004
- DOI: 10.1530/REP-14-0483
Med1 regulates meiotic progression during spermatogenesis in mice
Abstract
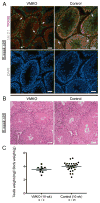
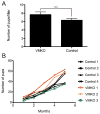
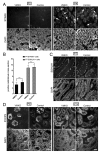
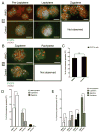
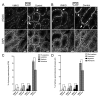
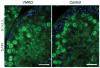
Spermatogenesis is a highly coordinated process. Signaling from nuclear hormone receptors, like those for retinoic acid (RA), is important for normal spermatogenesis. However, the mechanisms regulating these signals are poorly understood. Mediator complex subunit 1 (MED1) is a transcriptional enhancer that directly modulates transcription from nuclear hormone receptors. MED1 is present in male germ cells throughout mammalian development, but its function during spermatogenesis is unknown. To determine its role, we generated mice lacking Med1 specifically in their germ cells beginning just before birth. Conditional Med1 knockout males are fertile, exhibiting normal testis weights and siring ordinary numbers of offspring. RA-responsive gene products stimulated by RA gene 8 (Stra8) and synaptonemal complex protein 3 (Sycp3) are first detected in knockout spermatogonia at the expected time points during the first wave of spermatogenesis, and persist with normal patterns of cellular distribution in adult knockout testes. Meiotic progression, however, is altered in the absence of Med1. At postnatal day 7 (P7), zygotene-stage knockout spermatocytes are already detected, unlike in control testes, with fewer pre-leptotene-stage cells and more leptotene spermatocytes observed in the knockouts. At P9, Med1 knockout spermatocytes prematurely enter pachynema. Once formed, greater numbers of knockout spermatocytes remain in pachynema relative to the other stages of meiosis throughout testis development and its maintenance in the adult. Meiotic exit is not inhibited. We conclude that MED1 regulates the temporal progression of primary spermatocytes through meiosis, with its absence resulting in abbreviated pre-leptotene, leptotene, and zygotene stages, and a prolonged pachytene stage.
© 2015 Society for Reproduction and Fertility.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

Similar articles
-
The role of tyrosine phosphatase Shp2 in spermatogonial differentiation and spermatocyte meiosis.Asian J Androl. 2020 Jan-Feb;22(1):79-87. doi: 10.4103/aja.aja_49_19. Asian J Androl. 2020. PMID: 31210146 Free PMC article.
-
Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10132-E10141. doi: 10.1073/pnas.1710837114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109271 Free PMC article.
-
Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes.Biol Reprod. 2008 Jul;79(1):35-42. doi: 10.1095/biolreprod.107.066795. Epub 2008 Mar 5. Biol Reprod. 2008. PMID: 18322276 Free PMC article.
-
Conditional ablation of DIS3L2 ribonuclease in pre-meiotic germ cells causes defective spermatogenesis and infertility in male mice.Theranostics. 2024 Sep 3;14(14):5621-5642. doi: 10.7150/thno.98620. eCollection 2024. Theranostics. 2024. PMID: 39310107 Free PMC article.
-
Nociceptin and meiosis during spermatogenesis in postnatal testes.Vitam Horm. 2015;97:167-86. doi: 10.1016/bs.vh.2014.10.003. Epub 2015 Jan 14. Vitam Horm. 2015. PMID: 25677772 Review.
Cited by
-
Detection of genomic structural variations in Guizhou indigenous pigs and the comparison with other breeds.PLoS One. 2018 Mar 20;13(3):e0194282. doi: 10.1371/journal.pone.0194282. eCollection 2018. PLoS One. 2018. PMID: 29558483 Free PMC article.
-
Rhox13 is required for a quantitatively normal first wave of spermatogenesis in mice.Reproduction. 2016 Nov;152(5):379-88. doi: 10.1530/REP-16-0268. Epub 2016 Aug 2. Reproduction. 2016. PMID: 27486269 Free PMC article.
-
Knockout of cyclin-dependent kinases 8 and 19 leads to depletion of cyclin C and suppresses spermatogenesis and male fertility in mice.Elife. 2025 Apr 2;13:RP96465. doi: 10.7554/eLife.96465. Elife. 2025. PMID: 40172945 Free PMC article.
-
The role of tyrosine phosphatase Shp2 in spermatogonial differentiation and spermatocyte meiosis.Asian J Androl. 2020 Jan-Feb;22(1):79-87. doi: 10.4103/aja.aja_49_19. Asian J Androl. 2020. PMID: 31210146 Free PMC article.
-
Genetically Engineered Mice Unveil In Vivo Roles of the Mediator Complex.Int J Mol Sci. 2023 May 26;24(11):9330. doi: 10.3390/ijms24119330. Int J Mol Sci. 2023. PMID: 37298278 Free PMC article. Review.
References
-
- Ashley T. The mouse “tool box” for meiotic studies. Cytogenet Genome Res. 2004;105:166–171. - PubMed
-
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–167. - PubMed
-
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. - PubMed
-
- Burnicka-Turek O, Shirneshan K, Paprotta I, Grzmil P, Meinhardt A, Engel W, Adham IM. Inactivation of insulin-like factor 6 disrupts the progression of spermatogenesis at late meiotic prophase. Endocrinology. 2009;150:4348–4357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

