YAP is essential for tissue tension to ensure vertebrate 3D body shape
- PMID: 25778702
- PMCID: PMC4720436
- DOI: 10.1038/nature14215
YAP is essential for tissue tension to ensure vertebrate 3D body shape
Abstract
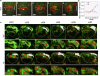
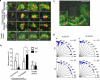
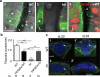
Vertebrates have a unique 3D body shape in which correct tissue and organ shape and alignment are essential for function. For example, vision requires the lens to be centred in the eye cup which must in turn be correctly positioned in the head. Tissue morphogenesis depends on force generation, force transmission through the tissue, and response of tissues and extracellular matrix to force. Although a century ago D'Arcy Thompson postulated that terrestrial animal body shapes are conditioned by gravity, there has been no animal model directly demonstrating how the aforementioned mechano-morphogenetic processes are coordinated to generate a body shape that withstands gravity. Here we report a unique medaka fish (Oryzias latipes) mutant, hirame (hir), which is sensitive to deformation by gravity. hir embryos display a markedly flattened body caused by mutation of YAP, a nuclear executor of Hippo signalling that regulates organ size. We show that actomyosin-mediated tissue tension is reduced in hir embryos, leading to tissue flattening and tissue misalignment, both of which contribute to body flattening. By analysing YAP function in 3D spheroids of human cells, we identify the Rho GTPase activating protein ARHGAP18 as an effector of YAP in controlling tissue tension. Together, these findings reveal a previously unrecognised function of YAP in regulating tissue shape and alignment required for proper 3D body shape. Understanding this morphogenetic function of YAP could facilitate the use of embryonic stem cells to generate complex organs requiring correct alignment of multiple tissues.
Figures

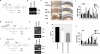

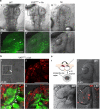

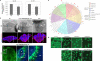

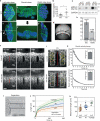


Comment in
-
Crumbling under pressure.Dev Cell. 2015 Apr 20;33(2):122-4. doi: 10.1016/j.devcel.2015.04.005. Dev Cell. 2015. PMID: 25898164
References
-
- Thompson DA. On Growth and Form. Cambridge University Press; 1917. pp. 1–1136.
-
- Furutani-Seiki M, et al. Neural degeneration mutants in the zebrafish, Danio rerio. Development. 1996;123:229–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

