Selection on noise constrains variation in a eukaryotic promoter
- PMID: 25778704
- PMCID: PMC4455047
- DOI: 10.1038/nature14244
Selection on noise constrains variation in a eukaryotic promoter
Abstract
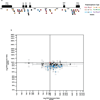
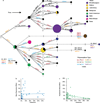
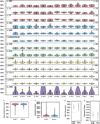
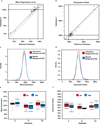
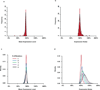
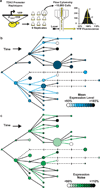
Genetic variation segregating within a species reflects the combined activities of mutation, selection, and genetic drift. In the absence of selection, polymorphisms are expected to be a random subset of new mutations; thus, comparing the effects of polymorphisms and new mutations provides a test for selection. When evidence of selection exists, such comparisons can identify properties of mutations that are most likely to persist in natural populations. Here we investigate how mutation and selection have shaped variation in a cis-regulatory sequence controlling gene expression by empirically determining the effects of polymorphisms segregating in the TDH3 promoter among 85 strains of Saccharomyces cerevisiae and comparing their effects to a distribution of mutational effects defined by 236 point mutations in the same promoter. Surprisingly, we find that selection on expression noise (that is, variability in expression among genetically identical cells) appears to have had a greater impact on sequence variation in the TDH3 promoter than selection on mean expression level. This is not necessarily because variation in expression noise impacts fitness more than variation in mean expression level, but rather because of differences in the distributions of mutational effects for these two phenotypes. This study shows how systematically examining the effects of new mutations can enrich our understanding of evolutionary mechanisms. It also provides rare empirical evidence of selection acting on expression noise.
Figures







References
Additional References for Methods section
-
- Taly J-F, et al. Using the T-Coffee package to build multiple sequence alignments of protein, RNA, DNA sequences and 3D structures. Nat. Protoc. 2011;6:1669–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

