A diversity-oriented synthesis strategy enabling the combinatorial-type variation of macrocyclic peptidomimetic scaffolds
- PMID: 25778821
- PMCID: PMC4441267
- DOI: 10.1039/c5ob00371g
A diversity-oriented synthesis strategy enabling the combinatorial-type variation of macrocyclic peptidomimetic scaffolds
Abstract
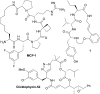
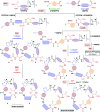
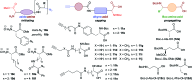
Macrocyclic peptidomimetics are associated with a broad range of biological activities. However, despite such potentially valuable properties, the macrocyclic peptidomimetic structural class is generally considered as being poorly explored within drug discovery. This has been attributed to the lack of general methods for producing collections of macrocyclic peptidomimetics with high levels of structural, and thus shape, diversity. In particular, there is a lack of scaffold diversity in current macrocyclic peptidomimetic libraries; indeed, the efficient construction of diverse molecular scaffolds presents a formidable general challenge to the synthetic chemist. Herein we describe a new, advanced strategy for the diversity-oriented synthesis (DOS) of macrocyclic peptidomimetics that enables the combinatorial variation of molecular scaffolds (core macrocyclic ring architectures). The generality and robustness of this DOS strategy is demonstrated by the step-efficient synthesis of a structurally diverse library of over 200 macrocyclic peptidomimetic compounds, each based around a distinct molecular scaffold and isolated in milligram quantities, from readily available building-blocks. To the best of our knowledge this represents an unprecedented level of scaffold diversity in a synthetically derived library of macrocyclic peptidomimetics. Cheminformatic analysis indicated that the library compounds access regions of chemical space that are distinct from those addressed by top-selling brand-name drugs and macrocyclic natural products, illustrating the value of our DOS approach to sample regions of chemical space underexploited in current drug discovery efforts. An analysis of three-dimensional molecular shapes illustrated that the DOS library has a relatively high level of shape diversity.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

