New insights into the mechanisms of gene electrotransfer--experimental and theoretical analysis
- PMID: 25778848
- PMCID: PMC5390920
- DOI: 10.1038/srep09132
New insights into the mechanisms of gene electrotransfer--experimental and theoretical analysis
Abstract
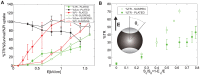
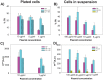
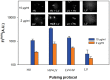
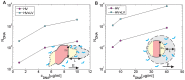
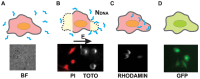
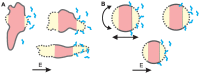
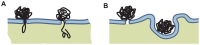
Gene electrotransfer is a promising non-viral method of gene delivery. In our in vitro study we addressed open questions about this multistep process: how electropermeabilization is related to electrotransfer efficiency; the role of DNA electrophoresis for contact and transfer across the membrane; visualization and theoretical analysis of DNA-membrane interaction and its relation to final transfection efficiency; and the differences between plated and suspended cells. Combinations of high-voltage and low-voltage pulses were used. We obtained that electrophoresis is required for the insertion of DNA into the permeabilized membrane. The inserted DNA is slowly transferred into the cytosol, and nuclear entry is a limiting factor for optimal transfection. The quantification and theoretical analysis of the crucial parameters reveals that DNA-membrane interaction (NDNA) increases with higher DNA concentration or with the addition of electrophoretic LV pulses while transfection efficiency reaches saturation. We explain the differences between the transfection of cell suspensions and plated cells due to the more homogeneous size, shape and movement of suspended cells. Our results suggest that DNA is either translocated through the stable electropores or enters by electo-stimulated endocytosis, possibly dependent on pulse parameters. Understanding of the mechanisms enables the selection of optimal electric protocols for specific applications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level.J Gene Med. 2013 May;15(5):169-81. doi: 10.1002/jgm.2706. J Gene Med. 2013. PMID: 23564663
-
Mechanisms involved in gene electrotransfer using high- and low-voltage pulses--an in vitro study.Bioelectrochemistry. 2009 Feb;74(2):265-71. doi: 10.1016/j.bioelechem.2008.09.002. Epub 2008 Sep 21. Bioelectrochemistry. 2009. PMID: 18930698
-
The role of electrically stimulated endocytosis in gene electrotransfer.Bioelectrochemistry. 2012 Feb;83:38-45. doi: 10.1016/j.bioelechem.2011.08.005. Epub 2011 Aug 23. Bioelectrochemistry. 2012. PMID: 21907005
-
Mechanism by which electroporation mediates DNA migration and entry into cells and targeted tissues.Methods Mol Biol. 2008;423:19-33. doi: 10.1007/978-1-59745-194-9_2. Methods Mol Biol. 2008. PMID: 18370188 Review.
-
New insights in the visualization of membrane permeabilization and DNA/membrane interaction of cells submitted to electric pulses.Biochim Biophys Acta. 2005 Aug 5;1724(3):248-54. doi: 10.1016/j.bbagen.2005.04.005. Epub 2005 Apr 21. Biochim Biophys Acta. 2005. PMID: 15878640 Review.
Cited by
-
Synergistic combinations of short high-voltage pulses and long low-voltage pulses enhance irreversible electroporation efficacy.Sci Rep. 2017 Nov 9;7(1):15123. doi: 10.1038/s41598-017-15494-3. Sci Rep. 2017. PMID: 29123231 Free PMC article.
-
Effect of degeneration stage on non-viral tissue transfection of rd10 retina ex vivo.Mol Ther Nucleic Acids. 2025 Jul 1;36(3):102616. doi: 10.1016/j.omtn.2025.102616. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40704026 Free PMC article.
-
A Novel Method for Controlled Gene Expression via Combined Bleomycin and Plasmid DNA Electrotransfer.Int J Mol Sci. 2019 Aug 19;20(16):4047. doi: 10.3390/ijms20164047. Int J Mol Sci. 2019. PMID: 31430949 Free PMC article.
-
Involvement of a Rac1-Dependent Macropinocytosis Pathway in Plasmid DNA Delivery by Electrotransfection.Mol Ther. 2017 Mar 1;25(3):803-815. doi: 10.1016/j.ymthe.2016.12.009. Epub 2017 Jan 24. Mol Ther. 2017. PMID: 28129959 Free PMC article.
-
Aluminum particles generated during millisecond electric pulse application enhance adenovirus-mediated gene transfer in L929 cells.Sci Rep. 2021 Sep 6;11(1):17725. doi: 10.1038/s41598-021-96781-y. Sci Rep. 2021. PMID: 34489497 Free PMC article.
References
-
- Weaver J. C. & Chizmadzhev Y. A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 41, 135–160 (1996).
-
- Titomirov A., Sukharev S. & Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta 1088, 131–134 (1991). - PubMed
-
- Heller R. et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 389, 225–228 (1996). - PubMed
-
- Aihara H. & Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867–870 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

