PAPC mediates self/non-self-distinction during Snail1-dependent tissue separation
- PMID: 25778923
- PMCID: PMC4362454
- DOI: 10.1083/jcb.201409026
PAPC mediates self/non-self-distinction during Snail1-dependent tissue separation
Abstract
Cleft-like boundaries represent a type of cell sorting boundary characterized by the presence of a physical gap between tissues. We studied the cleft-like ectoderm-mesoderm boundary in Xenopus laevis and zebrafish gastrulae. We identified the transcription factor Snail1 as being essential for tissue separation, showed that its expression in the mesoderm depends on noncanonical Wnt signaling, and demonstrated that it enables paraxial protocadherin (PAPC) to promote tissue separation through two novel functions. First, PAPC attenuates planar cell polarity signaling at the ectoderm-mesoderm boundary to lower cell adhesion and facilitate cleft formation. Second, PAPC controls formation of a distinct type of adhesive contact between mesoderm and ectoderm cells that shows properties of a cleft-like boundary at the single-cell level. It consists of short stretches of adherens junction-like contacts inserted between intermediate-sized contacts and large intercellular gaps. These roles of PAPC constitute a self/non-self-recognition mechanism that determines the site of boundary formation at the interface between PAPC-expressing and -nonexpressing cells.
© 2015 Luu et al.
Figures
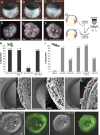

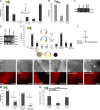


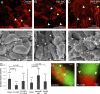
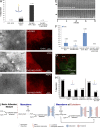



Comment in
-
Balancing cell behavior at boundaries.J Cell Biol. 2015 Mar 16;208(6):659-60. doi: 10.1083/jcb.201501107. J Cell Biol. 2015. PMID: 25778916 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

