Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice
- PMID: 25779632
- PMCID: PMC4387281
- DOI: 10.1084/jem.20132423
Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice
Abstract
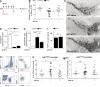
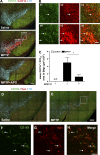
Neuroinflammation is increasingly recognized as a hallmark of neurodegeneration. Activated central nervous system-resident microglia and infiltrating immune cells contribute to the degeneration of dopaminergic neurons (DNs). However, how the inflammatory process leads to neuron loss and whether blocking this response would be beneficial to disease progression remains largely unknown. CD95 is a mediator of inflammation that has also been proposed as an apoptosis inducer in DNs, but previous studies using ubiquitous deletion of CD95 or CD95L in mouse models of neurodegeneration have generated conflicting results. Here we examine the role of CD95 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP)-induced neurodegeneration using tissue-specific deletion of CD95 or CD95L. We show that DN death is not mediated by CD95-induced apoptosis because deletion of CD95 in DNs does not influence MPTP-induced neurodegeneration. In contrast, deletion of CD95L in peripheral myeloid cells significantly protects against MPTP neurotoxicity and preserves striatal dopamine levels. Systemic pharmacological inhibition of CD95L dampens the peripheral innate response, reduces the accumulation of infiltrating myeloid cells, and efficiently prevents MPTP-induced DN death. Altogether, this study emphasizes the role of the peripheral innate immune response in neurodegeneration and identifies CD95 as potential pharmacological target for neurodegenerative disease.
© 2015 Gao et al.
Figures




References
-
- Brochard V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., et al. . 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 119:182–192 10.1172/JCI36470 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

