Conditional knockout of TMEM16A/anoctamin1 abolishes the calcium-activated chloride current in mouse vomeronasal sensory neurons
- PMID: 25779870
- PMCID: PMC4380210
- DOI: 10.1085/jgp.201411348
Conditional knockout of TMEM16A/anoctamin1 abolishes the calcium-activated chloride current in mouse vomeronasal sensory neurons
Abstract
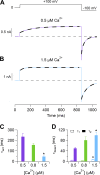
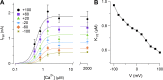
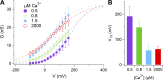
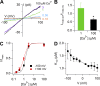
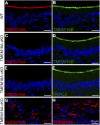
Pheromones are substances released from animals that, when detected by the vomeronasal organ of other individuals of the same species, affect their physiology and behavior. Pheromone binding to receptors on microvilli on the dendritic knobs of vomeronasal sensory neurons activates a second messenger cascade to produce an increase in intracellular Ca(2+) concentration. Here, we used whole-cell and inside-out patch-clamp analysis to provide a functional characterization of currents activated by Ca(2+) in isolated mouse vomeronasal sensory neurons in the absence of intracellular K(+). In whole-cell recordings, the average current in 1.5 µM Ca(2+) and symmetrical Cl(-) was -382 pA at -100 mV. Ion substitution experiments and partial blockade by commonly used Cl(-) channel blockers indicated that Ca(2+) activates mainly anionic currents in these neurons. Recordings from inside-out patches from dendritic knobs of mouse vomeronasal sensory neurons confirmed the presence of Ca(2+)-activated Cl(-) channels in the knobs and/or microvilli. We compared the electrophysiological properties of the native currents with those mediated by heterologously expressed TMEM16A/anoctamin1 or TMEM16B/anoctamin2 Ca(2+)-activated Cl(-) channels, which are coexpressed in microvilli of mouse vomeronasal sensory neurons, and found a closer resemblance to those of TMEM16A. We used the Cre-loxP system to selectively knock out TMEM16A in cells expressing the olfactory marker protein, which is found in mature vomeronasal sensory neurons. Immunohistochemistry confirmed the specific ablation of TMEM16A in vomeronasal neurons. Ca(2+)-activated currents were abolished in vomeronasal sensory neurons of TMEM16A conditional knockout mice, demonstrating that TMEM16A is an essential component of Ca(2+)-activated Cl(-) currents in mouse vomeronasal sensory neurons.
© 2015 Amjad et al.
Figures






Similar articles
-
Calcium-activated chloride channels in the apical region of mouse vomeronasal sensory neurons.J Gen Physiol. 2012 Jul;140(1):3-15. doi: 10.1085/jgp.201210780. J Gen Physiol. 2012. PMID: 22732308 Free PMC article.
-
Ca2+-activated Cl- currents in the murine vomeronasal organ enhance neuronal spiking but are dispensable for male-male aggression.J Biol Chem. 2018 Jun 29;293(26):10392-10403. doi: 10.1074/jbc.RA118.003153. Epub 2018 May 16. J Biol Chem. 2018. PMID: 29769308 Free PMC article.
-
TMEM16A and TMEM16B Modulate Pheromone-Evoked Action Potential Firing in Mouse Vomeronasal Sensory Neurons.eNeuro. 2021 Sep 15;8(5):ENEURO.0179-21.2021. doi: 10.1523/ENEURO.0179-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34433575 Free PMC article.
-
Anoctamin 2/TMEM16B: a calcium-activated chloride channel in olfactory transduction.Exp Physiol. 2012 Feb;97(2):193-9. doi: 10.1113/expphysiol.2011.058230. Epub 2011 Sep 2. Exp Physiol. 2012. PMID: 21890523 Review.
-
The physiological roles of anoctamin2/TMEM16B and anoctamin1/TMEM16A in chemical senses.Cell Calcium. 2024 Jun;120:102889. doi: 10.1016/j.ceca.2024.102889. Epub 2024 Apr 18. Cell Calcium. 2024. PMID: 38677213 Review.
Cited by
-
Mechanisms underlying pre- and postnatal development of the vomeronasal organ.Cell Mol Life Sci. 2021 Jun;78(12):5069-5082. doi: 10.1007/s00018-021-03829-3. Epub 2021 Apr 19. Cell Mol Life Sci. 2021. PMID: 33871676 Free PMC article. Review.
-
TRICK or TRP? What Trpc2(-/-) mice tell us about vomeronasal organ mediated innate behaviors.Front Neurosci. 2015 Jun 23;9:221. doi: 10.3389/fnins.2015.00221. eCollection 2015. Front Neurosci. 2015. PMID: 26157356 Free PMC article.
-
Slow Inactivation of Sodium Channels Contributes to Short-Term Adaptation in Vomeronasal Sensory Neurons.eNeuro. 2022 May 17;9(3):ENEURO.0471-21.2022. doi: 10.1523/ENEURO.0471-21.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35487703 Free PMC article.
-
Direct repression of anoctamin 1 (ANO1) gene transcription by Gli proteins.FASEB J. 2019 May;33(5):6632-6642. doi: 10.1096/fj.201802373R. Epub 2019 Feb 25. FASEB J. 2019. PMID: 30802137 Free PMC article.
-
Modulating Ca²⁺ signals: a common theme for TMEM16, Ist2, and TMC.Pflugers Arch. 2016 Mar;468(3):475-90. doi: 10.1007/s00424-015-1767-4. Epub 2015 Dec 23. Pflugers Arch. 2016. PMID: 26700940 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

