Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise
- PMID: 25780062
- PMCID: PMC4822524
- DOI: 10.1093/jnci/djv040
Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise
Abstract
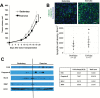
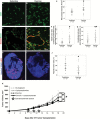
Exercise has been shown to improve postischemia perfusion of normal tissues; we investigated whether these effects extend to solid tumors. Estrogen receptor-negative (ER-, 4T1) and ER+ (E0771) tumor cells were implanted orthotopically into syngeneic mice (BALB/c, N = 11-12 per group) randomly assigned to exercise or sedentary control. Tumor growth, perfusion, hypoxia, and components of the angiogenic and apoptotic cascades were assessed by MRI, immunohistochemistry, western blotting, and quantitative polymerase chain reaction and analyzed with one-way and repeated measures analysis of variance and linear regression. All statistical tests were two-sided. Exercise statistically significantly reduced tumor growth and was associated with a 1.4-fold increase in apoptosis (sedentary vs exercise: 1544 cells/mm(2), 95% CI = 1223 to 1865 vs 2168 cells/mm(2), 95% CI = 1620 to 2717; P = .048), increased microvessel density (P = .004), vessel maturity (P = .006) and perfusion, and reduced intratumoral hypoxia (P = .012), compared with sedentary controls. We also tested whether exercise could improve chemotherapy (cyclophosphamide) efficacy. Exercise plus chemotherapy prolonged growth delay compared with chemotherapy alone (P < .001) in the orthotopic 4T1 model (n = 17 per group). Exercise is a potential novel adjuvant treatment of breast cancer.
© The Author 2015. Published by Oxford University Press.
Figures
References
-
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature Rev Drug Discov. 2011;10(6):417–427. - PubMed
-
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. - PubMed
-
- Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423(6940):593–595. - PubMed
-
- DeClerck K, Elble RC. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Fronti Biosci (Landmark Ed). 2010;15:213–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

