Cell kinetics during regeneration in the sponge Halisarca caerulea: how local is the response to tissue damage?
- PMID: 25780772
- PMCID: PMC4358696
- DOI: 10.7717/peerj.820
Cell kinetics during regeneration in the sponge Halisarca caerulea: how local is the response to tissue damage?
Abstract
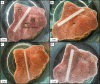
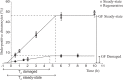
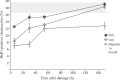
Sponges have a remarkable capacity to rapidly regenerate in response to wound infliction. In addition, sponges rapidly renew their filter systems (choanocytes) to maintain a healthy population of cells. This study describes the cell kinetics of choanocytes in the encrusting reef sponge Halisarca caerulea during early regeneration (0-8 h) following experimental wound infliction. Subsequently, we investigated the spatial relationship between regeneration and cell proliferation over a six-day period directly adjacent to the wound, 1 cm, and 3 cm from the wound. Cell proliferation was determined by the incorporation of 5-bromo-2'-deoxyuridine (BrdU). We demonstrate that during early regeneration, the growth fraction of the choanocytes (i.e., the percentage of proliferative cells) adjacent to the wound is reduced (7.0 ± 2.5%) compared to steady-state, undamaged tissue (46.6 ± 2.6%), while the length of the cell cycle remained short (5.6 ± 3.4 h). The percentage of proliferative choanocytes increased over time in all areas and after six days of regeneration choanocyte proliferation rates were comparable to steady-state tissue. Tissue areas farther from the wound had higher rates of choanocyte proliferation than areas closer to the wound, indicating that more resources are demanded from tissue in the immediate vicinity of the wound. There was no difference in the number of proliferative mesohyl cells in regenerative sponges compared to steady-state sponges. Our data suggest that the production of collagen-rich wound tissue is a key process in tissue regeneration for H. caerulea, and helps to rapidly occupy the bare substratum exposed by the wound. Regeneration and choanocyte renewal are competing and negatively correlated life-history traits, both essential to the survival of sponges. The efficient allocation of limited resources to these life-history traits has enabled the ecological success and diversification of sponges.
Keywords: Cell kinetics; Choanocyte turnover; Collagen; Immunohistochemistry; Modular integration; Regeneration; Sponges; Trade-off.
Conflict of interest statement
Brittany E. Alexander, Ronald Osinga and Jasper M. de Goeij are employees of Porifarma B.V., Poelbos, The Netherlands.
Figures

References
-
- Alexander BE, Liebrand K, Osinga R, Van der Geest HG, Admiraal W, Cleutjens JPM, Schutte B, Verheyen F, Ribes M, Van Loon E, de Goeij JM. Cell turnover and detritus production in marine sponges from tropical and temperate benthic ecosystems. PLoS ONE. 2014;9(10):e820. doi: 10.1371/journal.pone.0109486. - DOI - PMC - PubMed
-
- Bell JJ. Regeneration rates of a sublittoral demosponge. Journal of the Marine Biology Association UK. 2002;82:169–170. doi: 10.1017/S0025315402005465. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

