Voltage-gated sodium channels in the mammalian heart
- PMID: 25780798
- PMCID: PMC4355518
- DOI: 10.5339/gcsp.2014.58
Voltage-gated sodium channels in the mammalian heart
Abstract
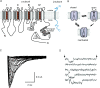
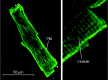
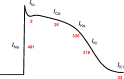
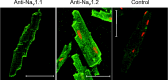
Mammalian species express nine functional voltage-gated Na(+) channels. Three of them, the cardiac-specific isoform Nav1.5 and the neuronal isoforms Nav1.8 and Nav1.9, are relatively resistant to the neurotoxin tetrodotoxin (TTX; IC50 ≥ 1 μM). The other six isoforms are highly sensitive to TTX with IC50 values in the nanomolar range. These isoforms are expressed in the central nervous system (Nav1.1, Nav1.2, Nav1.3, Nav1.6), in the skeletal muscle (Nav1.4), and in the peripheral nervous system (Nav1.6, Nav1.7). The isoform Nav1.5, encoded by the SCN5A gene, is responsible for the upstroke of the action potential in the heart. Mutations in SCN5A are associated with a variety of life-threatening arrhythmias, like long QT syndrome type 3 (LQT3), Brugada syndrome (BrS) or cardiac conduction disease (CCD). Previous immunohistochemical and electrophysiological assays demonstrated the cardiac expression of neuronal and skeletal muscle Na(+) channels in the heart of various mammals, which led to far-reaching speculations on their function. However, when comparing the Na(+) channel mRNA patterns in the heart of various mammalian species, only minute quantities of transcripts for TTX-sensitive Na(+) channels were detectable in whole pig and human hearts, suggesting that these channels are not involved in cardiac excitation phenomena in higher mammals. This conclusion is strongly supported by the fact that mutations in TTX-sensitive Na(+) channels were associated with epilepsy or skeletal muscle diseases, rather than with a pathological cardiac phenotype. Moreover, previous data from TTX-intoxicated animals and from cases of human tetrodotoxication showed that low TTX dosages caused at most little alterations of both the cardiac output and the electrocardiogram. Recently, genome-wide association studies identified SCN10A, the gene encoding Nav1.8, as a determinant of cardiac conduction parameters, and mutations in SCN10A have been associated with BrS. These novel findings opened a fascinating new research area in the cardiac ion channel field, and the on-going debate on how SCN10A/Nav1.8 affects cardiac conduction is very exciting.
Figures




References
-
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. - PubMed
-
- Rook MB, Evers MM, Vos MA, Bierhuizen MF. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res. 2012;93:12–23. - PubMed
-
- Patton DE, Isom LL, Catterall WA, Goldin AL. The adult rat brain beta 1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J Biol Chem. 1994;269:17649–17655. - PubMed
-
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. Beta 3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci U S A. 2000;97:2308–2313. - PMC - PubMed
-
- Zimmer T, Biskup C, Bollensdorff C, Benndorf K. The beta1 subunit but not the beta2 subunit colocalizes with the human heart Na+ channel (hH1) already within the endoplasmic reticulum. J Membr Biol. 2002;186:13–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
