Micellar electrokinetic chromatography method for measuring amino acid secretions from islets of Langerhans
- PMID: 25780900
- PMCID: PMC4433432
- DOI: 10.1002/elps.201400569
Micellar electrokinetic chromatography method for measuring amino acid secretions from islets of Langerhans
Abstract
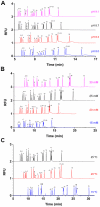
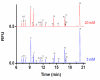
Islets of Langerhans are responsible for maintaining glucose homeostasis through regulated secretion of hormones and other factors. It is hypothesized that amino acids secreted from islets play a critical role in cell functionality and viability. For example, glutamate and gamma-aminobutyric acid have been proposed to work as paracrine signaling molecules within islets to coordinate the release of hormone secretion; other amino acids, such as glutamine, leucine, alanine, and arginine, have been shown to stimulate or potentiate glucose-stimulated insulin secretion. To characterize the potential roles that these small molecules may play in islet physiology, derivatization of amino acids in high-salt buffers commonly used in islet experiments with naphthalene-2,3-dicarboxaldehyde and MEKC separation conditions were optimized. The optimized conditions used d-norvaline as the internal standard and allowed quantification of 14 amino acids with LODs ranging from 0.2 to 7 nM. The RSDs of the migration times were 0.04-0.54% and the RSDs of the peak areas were 0.2-5.8% for the various amino acids. The effects of glucose and 2,4-dinitrophenol on amino acid secretions from islets were tested and a suppressive effect of glucose on gamma-aminobutyric acid release was observed, likely acting through adenosine triphosphate inactivation of glutamate decarboxylase.
Keywords: 2,4-Dinitrophenol (DNP); CE; Glucose; LIF; Naphthalene-2,3-dicarboxaldehyde (NDA).
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
Analysis of D-amino acids secreted from murine islets of Langerhans using Marfey's reagent and reversed phase LC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Dec 1;1231:123928. doi: 10.1016/j.jchromb.2023.123928. Epub 2023 Nov 10. J Chromatogr B Analyt Technol Biomed Life Sci. 2023. PMID: 37976942 Free PMC article.
-
Microfluidic Device for the Measurement of Amino Acid Secretion Dynamics from Murine and Human Islets of Langerhans.Anal Chem. 2016 Mar 15;88(6):3369-75. doi: 10.1021/acs.analchem.6b00071. Epub 2016 Feb 26. Anal Chem. 2016. PMID: 26891222
-
Stacking and separation of fluorescent derivatives of amino acids by micellar electrokinetic chromatography in the presence of poly(ethylene oxide).J Chromatogr A. 2007 Mar 30;1146(1):118-24. doi: 10.1016/j.chroma.2007.01.113. Epub 2007 Feb 4. J Chromatogr A. 2007. PMID: 17300792
-
The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion.Front Endocrinol (Lausanne). 2013 Dec 31;4:199. doi: 10.3389/fendo.2013.00199. Front Endocrinol (Lausanne). 2013. PMID: 24427154 Free PMC article. Review.
-
Microfluidic Devices for the Measurement of Cellular Secretion.Annu Rev Anal Chem (Palo Alto Calif). 2016 Jun 12;9(1):249-69. doi: 10.1146/annurev-anchem-071114-040409. Annu Rev Anal Chem (Palo Alto Calif). 2016. PMID: 27306310 Review.
Cited by
-
Small molecules released from islets of Langerhans determined by liquid chromatography - mass spectrometry.Anal Methods. 2022 Jun 1;14(21):2100-2107. doi: 10.1039/d2ay00402j. Anal Methods. 2022. PMID: 35567801 Free PMC article.
-
Chiral micellar electrokinetic chromatographic separation for determination of L- and D-primary amines released from murine islets of Langerhans.Anal Methods. 2019 Mar 7;11(9):1276-1283. doi: 10.1039/c8ay02471e. Epub 2019 Feb 18. Anal Methods. 2019. PMID: 31073338 Free PMC article.
-
Analysis of D-amino acids secreted from murine islets of Langerhans using Marfey's reagent and reversed phase LC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Dec 1;1231:123928. doi: 10.1016/j.jchromb.2023.123928. Epub 2023 Nov 10. J Chromatogr B Analyt Technol Biomed Life Sci. 2023. PMID: 37976942 Free PMC article.
-
3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling.Lab Chip. 2017 Jan 17;17(2):341-349. doi: 10.1039/c6lc01201a. Lab Chip. 2017. PMID: 27990542 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

