Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors
- PMID: 25781000
- PMCID: PMC4457193
- DOI: 10.1113/JP270045
Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors
Abstract
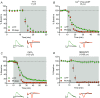
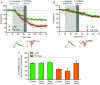
Cortical inputs to the dorsolateral striatum (DLS) are dynamically regulated during skill learning and habit formation, and are dysregulated in disorders characterized by impaired action control. Therefore, a mechanistic investigation of the processes regulating corticostriatal transmission is key to understanding DLS-associated circuit function, behaviour and pathology. Presynaptic GABA(B) and group II metabotropic glutamate (mGlu2/3) receptors exert marked inhibitory control over corticostriatal glutamate release in the DLS, yet the signalling pathways through which they do so are unclear. We developed a novel approach using the genetically encoded calcium (Ca(2+) ) indicator GCaMP6 to assess presynaptic Ca(2+) in corticostriatal projections to the DLS. Using simultaneous photometric presynaptic Ca(2+) and striatal field potential recordings, we report that relative to P/Q-type Ca(2+) channels, N-type channels preferentially contributed to evoked presynaptic Ca(2+) influx in motor cortex projections to, and excitatory transmission in, the DLS. Activation of GABA(B) or mGlu2/3 receptors inhibited both evoked presynaptic Ca(2+) transients and striatal field potentials. mGlu2/3 receptor-mediated depression did not require functional N-type Ca(2+) channels, but was attenuated by blockade of P/Q-type channels. These findings reveal presynaptic mechanisms of inhibitory modulation of corticostriatal function that probably contribute to the selection and shaping of behavioural repertoires.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures




Similar articles
-
Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses.J Neurosci. 2002 Jun 1;22(11):4346-56. doi: 10.1523/JNEUROSCI.22-11-04346.2002. J Neurosci. 2002. PMID: 12040040 Free PMC article.
-
N-type calcium channels mediate a GABA(B) presynaptic modulation in the corticostriatal synapse in turtle's paleostriatum augmentatum.Synapse. 2009 Oct;63(10):855-62. doi: 10.1002/syn.20667. Synapse. 2009. PMID: 19562696
-
Mechanism underlying unaltered cortical inhibitory synaptic transmission in contrast with enhanced excitatory transmission in CaV2.1 knockin migraine mice.Neurobiol Dis. 2014 Sep;69(100):225-34. doi: 10.1016/j.nbd.2014.05.035. Epub 2014 Jun 5. Neurobiol Dis. 2014. PMID: 24907493 Free PMC article.
-
Activity-dependent regulation of synaptic vesicle exocytosis and presynaptic short-term plasticity.Neurosci Res. 2011 May;70(1):16-23. doi: 10.1016/j.neures.2011.03.005. Epub 2011 Mar 29. Neurosci Res. 2011. PMID: 21453732 Review.
-
Presynaptic modulation of GABA release in the basal ganglia.Prog Brain Res. 2007;160:245-59. doi: 10.1016/S0079-6123(06)60014-9. Prog Brain Res. 2007. PMID: 17499118 Review.
Cited by
-
Circuit dysfunctions of associative and sensorimotor basal ganglia loops in alcohol use disorder: insights from animal models.Addict Neurosci. 2023 Mar;5:100056. doi: 10.1016/j.addicn.2022.100056. Epub 2022 Dec 8. Addict Neurosci. 2023. PMID: 36567745 Free PMC article.
-
Distinct sub-second dopamine signaling in dorsolateral striatum measured by a genetically-encoded fluorescent sensor.Nat Commun. 2023 Sep 22;14(1):5915. doi: 10.1038/s41467-023-41581-3. Nat Commun. 2023. PMID: 37739964 Free PMC article.
-
Heterosynaptic GABAB Receptor Function within Feedforward Microcircuits Gates Glutamatergic Transmission in the Nucleus Accumbens Core.J Neurosci. 2019 Nov 20;39(47):9277-9293. doi: 10.1523/JNEUROSCI.1395-19.2019. Epub 2019 Oct 2. J Neurosci. 2019. PMID: 31578230 Free PMC article.
-
Operant self-stimulation of thalamic terminals in the dorsomedial striatum is constrained by metabotropic glutamate receptor 2.Neuropsychopharmacology. 2020 Aug;45(9):1454-1462. doi: 10.1038/s41386-020-0626-y. Epub 2020 Jan 29. Neuropsychopharmacology. 2020. PMID: 31995814 Free PMC article.
-
Astrocytes in cocaine addiction and beyond.Mol Psychiatry. 2022 Jan;27(1):652-668. doi: 10.1038/s41380-021-01080-7. Epub 2021 Apr 9. Mol Psychiatry. 2022. PMID: 33837268 Free PMC article. Review.
References
-
- Allen MD. Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348:716–721. - PubMed
-
- Akopian G, Musleh W, Smith R. Walsh JP. Functional state of corticostriatal synapses determines their expression of short- and long-term plasticity. Synapse. 2000;38:271–280. - PubMed
-
- Amaral DG. Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

