Analysis of resistance to antimicrobials and presence of virulence/stress response genes in Campylobacter isolates from patients with severe diarrhoea
- PMID: 25781009
- PMCID: PMC4363897
- DOI: 10.1371/journal.pone.0119268
Analysis of resistance to antimicrobials and presence of virulence/stress response genes in Campylobacter isolates from patients with severe diarrhoea
Abstract
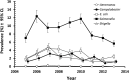
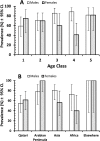
Campylobacter infections are a major cause of diarrhoea world-wide and two of the antimicrobials used for their control (erythromycin and ciprofloxacin) have been losing efficacy in recent years. In a sample of 174 genotyped isolates from the stools of patients with severe diarrhoea in Qatar, collected between 2005 and 2012, 63.2% showed resistance to ciprofloxacin, 8.6% to erythromycin, 0.57% to chloramphenicol and all were sensitive to gentamycin. While 33.9% of isolates were sensitive to all four antimicrobials, 59.8% were resistant to at least one, 6.3% were resistant to two and none showed resistance to three antimicrobials. There was no host sex- or age-dependence among isolates resistant to ciprofloxacin and erythromycin and no significant variation was found with the region of origin of the patients. All isolates were screened for the presence of 3 virulence factors (ciaB, cadF and cdtB) and two stress-response factors (htrB and clpP), all of which were present in more than 50% of the isolates. Host sex-, age- and region of origin-dependent variations in prevalence were found for some of these factors. Data analysis for the combination of virulence factors and their effect on antimicrobial resistance indicated that the prevalence of resistance to both erythromycin and ciprofloxacin was higher in isolates harbouring ciaB but not clpP. Prevalence of resistance to ciprofloxacin was similar in clpP positive and negative isolates also possessing htrB, while for htrB-negative isolates prevalence was higher in the absence of clpP. These results are discussed and their implications are highlighted.
Conflict of interest statement
Figures

References
-
- Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda DA, et al. Campylobacter. Vet Res. 2005; 36: 351–382. - PubMed
-
- Cover TL, Perez-Perez GI, Blaser MJ. Evaluation of cytotoxic activity in fecal filtrates from patients with Campylobacter jejuni or Campylobacter coli enteritis. FEMS Microbiol Lett. 1990; 58: 301–304. - PubMed
-
- Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007; 5: 665–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

