Design and mechanism of tetrahydrothiophene-based γ-aminobutyric acid aminotransferase inactivators
- PMID: 25781189
- PMCID: PMC4390550
- DOI: 10.1021/jacs.5b01155
Design and mechanism of tetrahydrothiophene-based γ-aminobutyric acid aminotransferase inactivators
Abstract
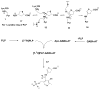
Low levels of γ-aminobutyric acid (GABA), one of two major neurotransmitters that regulate brain neuronal activity, are associated with many neurological disorders, such as epilepsy, Parkinson's disease, Alzheimer's disease, Huntington's disease, and cocaine addiction. One of the main methods to raise the GABA level in human brain is to use small molecules that cross the blood-brain barrier and inhibit the activity of γ-aminobutyric acid aminotransferase (GABA-AT), the enzyme that degrades GABA. We have designed a series of conformationally restricted tetrahydrothiophene-based GABA analogues with a properly positioned leaving group that could facilitate a ring-opening mechanism, leading to inactivation of GABA-AT. One compound in the series is 8 times more efficient an inactivator of GABA-AT than vigabatrin, the only FDA-approved inactivator of GABA-AT. Our mechanistic studies show that the compound inactivates GABA-AT by a new mechanism. The metabolite resulting from inactivation does not covalently bind to amino acid residues of GABA-AT but stays in the active site via H-bonding interactions with Arg-192, a π-π interaction with Phe-189, and a weak nonbonded S···O═C interaction with Glu-270, thereby inactivating the enzyme.
Figures
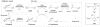
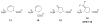







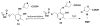

Similar articles
-
Design and Mechanism of GABA Aminotransferase Inactivators. Treatments for Epilepsies and Addictions.Chem Rev. 2018 Apr 11;118(7):4037-4070. doi: 10.1021/acs.chemrev.8b00009. Epub 2018 Mar 23. Chem Rev. 2018. PMID: 29569907 Free PMC article. Review.
-
Design and Mechanism of (S)-3-Amino-4-(difluoromethylenyl)cyclopent-1-ene-1-carboxylic Acid, a Highly Potent γ-Aminobutyric Acid Aminotransferase Inactivator for the Treatment of Addiction.J Am Chem Soc. 2018 Feb 14;140(6):2151-2164. doi: 10.1021/jacs.7b10965. Epub 2018 Jan 30. J Am Chem Soc. 2018. PMID: 29381352 Free PMC article.
-
Synthesis and evaluation of novel heteroaromatic substrates of GABA aminotransferase.Bioorg Med Chem. 2012 Oct 1;20(19):5763-73. doi: 10.1016/j.bmc.2012.08.009. Epub 2012 Aug 16. Bioorg Med Chem. 2012. PMID: 22944334 Free PMC article.
-
Mechanism of Inactivation of GABA Aminotransferase by (E)- and (Z)-(1S,3S)-3-Amino-4-fluoromethylenyl-1-cyclopentanoic Acid.ACS Chem Biol. 2015 Sep 18;10(9):2087-98. doi: 10.1021/acschembio.5b00212. Epub 2015 Jul 6. ACS Chem Biol. 2015. PMID: 26110556 Free PMC article.
-
Design of potential anticonvulsant agents: mechanistic classification of GABA aminotransferase inactivators.J Med Chem. 1989 Nov;32(11):2413-21. doi: 10.1021/jm00131a001. J Med Chem. 1989. PMID: 2681782 Review.
Cited by
-
QSAR and Molecular Docking Studies of the Inhibitory Activity of Novel Heterocyclic GABA Analogues over GABA-AT.Molecules. 2018 Nov 15;23(11):2984. doi: 10.3390/molecules23112984. Molecules. 2018. PMID: 30445747 Free PMC article.
-
From TgO/GABA-AT, GABA, and T-263 Mutant to Conception of Toxoplasma.iScience. 2023 Nov 16;27(1):108477. doi: 10.1016/j.isci.2023.108477. eCollection 2024 Jan 19. iScience. 2023. PMID: 38205261 Free PMC article.
-
Novel-Substituted Heterocyclic GABA Analogues. Enzymatic Activity against the GABA-AT Enzyme from Pseudomonas fluorescens and In Silico Molecular Modeling.Molecules. 2018 May 9;23(5):1128. doi: 10.3390/molecules23051128. Molecules. 2018. PMID: 29747438 Free PMC article.
-
Structural and Kinetic Analyses Reveal the Dual Inhibition Modes of Ornithine Aminotransferase by (1S,3S)-3-Amino-4-(hexafluoropropan-2-ylidenyl)-cyclopentane-1-carboxylic Acid (BCF3).ACS Chem Biol. 2021 Jan 15;16(1):67-75. doi: 10.1021/acschembio.0c00728. Epub 2020 Dec 14. ACS Chem Biol. 2021. PMID: 33316155 Free PMC article.
-
Design and Mechanism of GABA Aminotransferase Inactivators. Treatments for Epilepsies and Addictions.Chem Rev. 2018 Apr 11;118(7):4037-4070. doi: 10.1021/acs.chemrev.8b00009. Epub 2018 Mar 23. Chem Rev. 2018. PMID: 29569907 Free PMC article. Review.
References
-
- Bromfield EB, Cavazos JE, Sirven JI, editors. An Introduction to Epilepsy [Internet] American Epilepsy Society; West Hartford, CT: 2006. Chapter 1: Basic Mechanisms Underlying Seizures and Epilepsy.
-
- Epilepsy Foundation. [accessed Jul 29, 2014];About Epilepsy: The Basics. http://www.epilepsy.com/learn/about-epilepsy-basics.
-
- Karlsson A, Fonnum F, Malthe-Sørenssen D, Storm-Mathisen J. Biochem Pharmacol. 1974;23:3053–3061. - PubMed
-
- Baxter CF, Roberts E. J Biol Chem. 1958;233:1135–1139. - PubMed
-
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Mol Brain Res. 1995;33:7–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

