Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence
- PMID: 25781230
- PMCID: PMC4354350
- DOI: 10.1038/tp.2015.20
Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence
Abstract
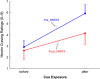
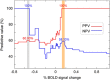
Injectable extended-release naltrexone (XRNTX) presents an effective therapeutic strategy for opioid addiction, however its utility could be hampered by poor adherence. To gain a better insight into this phenomenon, we utilized blood oxygenation level-dependent functional magnetic resonance imaging (fMRI) in conjunction with a validated cue-induced craving procedure to examine neural correlates of XRNTX adherence. We operationalized treatment adherence as the number of monthly XRNTX injections (range: 0-3) administered to a group of fully detoxified heroin-dependent subjects (n=32). Additional outcomes included urine toxicology screening and self-reported tobacco use. The presented heroin-related visual cues reliably elicited heroin craving in all tested subjects. Nine, five, three and 15 of the participants, respectively, received zero, one, two and three XRNTX injections, predicted by the individual baseline fMRI signal change in response to the cues in the medial prefrontal cortex, a brain region involved in inhibitory self-control and emotional appraisal. The incidence of opioid-positive urines during the XRNTX therapy was low and remained about half the pre-treatment rate after the XRNTX ended. During the treatment, cigarette smoking behaviors followed patterns of opioid use, while cocaine consumption was increased with reductions in opioid use. The present data support the hypothesis that medial prefrontal cortex functions are involved in adherence to opioid antagonist therapy. A potential role of concurrent non-opioid addictive substances consumption during the XRNTX pharmacotherapy warrants further investigation. Our findings set the stage for further bio-behavioral investigations of the mechanisms of relapse prevention in opioid dependence.
Figures



References
-
- Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–1347. - PubMed
-
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–2066. - PubMed
-
- SAMHSA . Substance Abuse and Mental Health Services Administration: Rockville, MD, USA; 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings.
-
- NIDA . Research Report Series. National Institutes of Health: Bethesda, MD, USA; 2014. Heroin.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

