Tat-antioxidant 1 protects against stress-induced hippocampal HT-22 cells death and attenuate ischaemic insult in animal model
- PMID: 25781353
- PMCID: PMC4459847
- DOI: 10.1111/jcmm.12513
Tat-antioxidant 1 protects against stress-induced hippocampal HT-22 cells death and attenuate ischaemic insult in animal model
Abstract
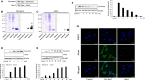
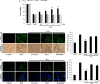
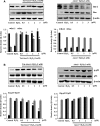
Oxidative stress-induced reactive oxygen species (ROS) are responsible for various neuronal diseases. Antioxidant 1 (Atox1) regulates copper homoeostasis and promotes cellular antioxidant defence against toxins generated by ROS. The roles of Atox1 protein in ischaemia, however, remain unclear. In this study, we generated a protein transduction domain fused Tat-Atox1 and examined the roles of Tat-Atox1 in oxidative stress-induced hippocampal HT-22 cell death and an ischaemic injury animal model. Tat-Atox1 effectively transduced into HT-22 cells and it protected cells against the effects of hydrogen peroxide (H2O2)-induced toxicity including increasing of ROS levels and DNA fragmentation. At the same time, Tat-Atox1 regulated cellular survival signalling such as p53, Bad/Bcl-2, Akt and mitogen-activate protein kinases (MAPKs). In the animal ischaemia model, transduced Tat-Atox1 protected against neuronal cell death in the hippocampal CA1 region. In addition, Tat-Atox1 significantly decreased the activation of astrocytes and microglia as well as lipid peroxidation in the CA1 region after ischaemic insult. Taken together, these results indicate that transduced Tat-Atox1 protects against oxidative stress-induced HT-22 cell death and against neuronal damage in animal ischaemia model. Therefore, we suggest that Tat-Atox1 has potential as a therapeutic agent for the treatment of oxidative stress-induced ischaemic damage.
Keywords: Tat-Atox1; ischaemic injury; oxidative stress; protein therapy; protein transduction domain.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Tat-NOL3 protects against hippocampal neuronal cell death induced by oxidative stress through the regulation of apoptotic pathways.Int J Mol Med. 2016 Jul;38(1):225-35. doi: 10.3892/ijmm.2016.2596. Epub 2016 May 19. Int J Mol Med. 2016. PMID: 27221790
-
Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury.Free Radic Biol Med. 2014 Feb;67:195-210. doi: 10.1016/j.freeradbiomed.2013.10.815. Epub 2013 Nov 16. Free Radic Biol Med. 2014. PMID: 24252591
-
Effects of low doses of Tat-PIM2 protein against hippocampal neuronal cell survival.J Neurol Sci. 2015 Nov 15;358(1-2):226-35. doi: 10.1016/j.jns.2015.08.1549. Epub 2015 Sep 2. J Neurol Sci. 2015. PMID: 26365288
-
Copper chaperone antioxidant 1: multiple roles and a potential therapeutic target.J Mol Med (Berl). 2023 May;101(5):527-542. doi: 10.1007/s00109-023-02311-w. Epub 2023 Apr 5. J Mol Med (Berl). 2023. PMID: 37017692 Review.
-
Mithramycin, an agent for developing new therapeutic drugs for neurodegenerative diseases.J Pharmacol Sci. 2013;122(4):251-6. doi: 10.1254/jphs.13r02cp. Epub 2013 Jul 30. J Pharmacol Sci. 2013. PMID: 23902990 Review.
Cited by
-
Transduced Tat-aldose Reductase Protects Hippocampal Neuronal Cells against Oxidative Stress-induced Damage.Exp Neurobiol. 2019 Oct 31;28(5):612-627. doi: 10.5607/en.2019.28.5.612. Exp Neurobiol. 2019. PMID: 31698553 Free PMC article.
-
Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans.BMC Complement Altern Med. 2017 Dec 28;17(1):551. doi: 10.1186/s12906-017-2050-3. BMC Complement Altern Med. 2017. PMID: 29282044 Free PMC article.
-
Tat-indoleamine 2,3-dioxygenase 1 elicits neuroprotective effects on ischemic injury.BMB Rep. 2020 Nov;53(11):582-587. doi: 10.5483/BMBRep.2020.53.11.114. BMB Rep. 2020. PMID: 32684242 Free PMC article.
-
Neuroprotective Effect of 3-(Naphthalen-2-Yl(Propoxy)Methyl)Azetidine Hydrochloride on Brain Ischaemia/Reperfusion Injury.J Neuroimmune Pharmacol. 2017 Sep;12(3):447-461. doi: 10.1007/s11481-017-9733-x. Epub 2017 Feb 28. J Neuroimmune Pharmacol. 2017. PMID: 28247179
-
The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution.Antioxidants (Basel). 2016 Jul 27;5(3):25. doi: 10.3390/antiox5030025. Antioxidants (Basel). 2016. PMID: 27472369 Free PMC article. Review.
References
-
- Hung IH, Casareno RL, Labesse G, et al. HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense. J Biol Chem. 1998;273:1749–54. - PubMed
-
- Palm-Espling ME, Niemiec MS, Wittung-Stafshede P. Role of metal in folding and stability of copper proteins in vitro. Biochim Biophys Acta. 2012;1823:1594–603. - PubMed
-
- Moore SD, Helmle KE, Prat LM, et al. Tissue localization of the copper chaperone ATOX1 and its potential role in disease. Mamm Genome. 2002;13:563–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

