Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial
- PMID: 25781618
- PMCID: PMC4363688
- DOI: 10.1371/journal.pone.0118174
Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial
Abstract
Background: Recent studies suggest that vitamin D deficiency represents an additional cofactor of renal anemia, with several mechanisms accounting for this relationship. In line with it, the administration of vitamin D or its analogues has been associated with an improvement of anemia. There are no data, however, about a direct effect of paricalcitol on hemoglobin (Hb) levels. Therefore, we conducted a study to determine whether paricalcitol, compared to calcitriol, improves anemia in patients with chronic kidney disease (CKD).
Methods: In this randomized trial 60 CKD patients stage 3b-5 and anemia (Hb levels: 10-12.5 g/dL) were assigned (1:1) to receive low doses of calcitriol (Group Calcitriol) or paricalcitol (Group Paricalcitol) for 6 months. All the patients had normal values of plasma calcium, phosphorus and PTH, a stable iron balance, and normal values of C-Reactive Protein. The primary endpoint was to evaluate the effects of the two treatments on Hb levels; the modifications in 24hr-proteinuria (UProt) were also evaluated.
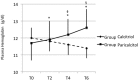
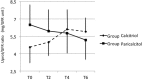
Results: A significant Group x Time interaction effect was observed in the longitudinal analysis of Hb levels (F(1,172)=31.4, p<0.001). Subjects in Paricalcitol experienced a significant monthly increase of Hb levels equal to +0.16 g/dL [95% C.I. 0.10 to +0.22, p<0.001) while in Group Calcitriol, Hb decrease throughout the follow-up with an average monthly rate of -0.10 g/dL (95% C.I.: -0.17 to -0.04, p<0.001). In Group Paricalcitol, UProt was significantly reduced after 6 months [0.35 (0.1-1.2) vs 0.59 (0.2-1.6), p<0.01], whereas no significant difference emerged in Group Calcitriol. Plasma levels of calcium, phosphate, PTH and of inflammation markers remained in the normal range in both groups throughout the study.
Conclusions: Short-term exposure to paricalcitol results in an independent increase in Hb levels, which occurred with no modification of iron balance, inflammatory markers, and PTH plasma concentrations, and was associated with a decrease in UProt.
Trial registration: ClinicalTrials.gov NCT01768351.
Conflict of interest statement
Figures

Similar articles
-
Effect of paricalcitol on mineral bone metabolism in kidney transplant recipients with secondary hyperparathyroidism.Nefrologia. 2015;35(4):363-73. doi: 10.1016/j.nefro.2015.06.018. Epub 2015 Aug 4. Nefrologia. 2015. PMID: 26306956 English, Spanish.
-
Paricalcitol reduces proteinuria but does not modify peritoneal protein loss in patients on peritoneal dialysis.Nefrologia. 2013 Jan 18;33(1):70-6. doi: 10.3265/Nefrologia.pre2012.Oct.11635. Nefrologia. 2013. PMID: 23364628 English, Spanish.
-
A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3-4 CKD.Clin J Am Soc Nephrol. 2014 Sep 5;9(9):1620-6. doi: 10.2215/CJN.10661013. Epub 2014 Jun 26. Clin J Am Soc Nephrol. 2014. PMID: 24970869 Free PMC article. Clinical Trial.
-
Paricalcitol, a new agent for the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis.Clin Ther. 1999 Mar;21(3):432-41. doi: 10.1016/S0149-2918(00)88299-5. Clin Ther. 1999. PMID: 10321413 Review.
-
Vitamin D, vitamin D receptor and the importance of its activation in patients with chronic kidney disease.Nefrologia. 2015;35(1):28-41. doi: 10.3265/Nefrologia.pre2014.Sep.11796. Nefrologia. 2015. PMID: 25611831 English, Spanish.
Cited by
-
Sodium-Glucose Co-transporter-2 Inhibitors and Nephroprotection in Diabetic Patients: More Than a Challenge.Front Med (Lausanne). 2021 Jun 4;8:654557. doi: 10.3389/fmed.2021.654557. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34150796 Free PMC article. Review.
-
Vitamin D Status and the Risk of Anemia in Community-Dwelling Adults: Results from the National Health and Nutrition Examination Survey 2001-2006.Medicine (Baltimore). 2015 Dec;94(50):e1799. doi: 10.1097/MD.0000000000001799. Medicine (Baltimore). 2015. PMID: 26683908 Free PMC article.
-
High-Dose Vitamin D3 Administration Is Associated With Increases in Hemoglobin Concentrations in Mechanically Ventilated Critically Ill Adults: A Pilot Double-Blind, Randomized, Placebo-Controlled Trial.JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):87-94. doi: 10.1177/0148607116678197. Epub 2017 Dec 11. JPEN J Parenter Enteral Nutr. 2018. PMID: 29505145 Free PMC article. Clinical Trial.
-
Raising awareness on the therapeutic role of cholecalciferol in CKD: a multidisciplinary-based opinion.Endocrine. 2018 Feb;59(2):242-259. doi: 10.1007/s12020-017-1369-3. Epub 2017 Jul 19. Endocrine. 2018. PMID: 28726185 Free PMC article. Review.
-
Vitamin D Supplementation for Patients with Chronic Kidney Disease: A Systematic Review and Meta-analyses of Trials Investigating the Response to Supplementation and an Overview of Guidelines.Calcif Tissue Int. 2021 Aug;109(2):157-178. doi: 10.1007/s00223-021-00844-1. Epub 2021 Apr 25. Calcif Tissue Int. 2021. PMID: 33895867 Free PMC article.
References
-
- Lin R, White JH. The pleiotropic effects of Vitamin D. BioEssays 2003; 26: 21–28. - PubMed
-
- Nagpal S, Na SN, Rathrachelam R. Noncalcemic actions of Vitamin D Receptor ligands. Endocr Rev 2005; 26: 662–687. - PubMed
-
- Lee JH O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency, an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008; 52: 1949–1956. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

