The receptor for advanced glycation end products (RAGE) contributes to the progression of emphysema in mice
- PMID: 25781626
- PMCID: PMC4364508
- DOI: 10.1371/journal.pone.0118979
The receptor for advanced glycation end products (RAGE) contributes to the progression of emphysema in mice
Abstract
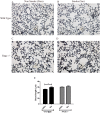
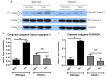
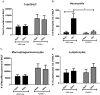
Several recent clinical studies have implied a role for the receptor for advanced glycation end products (RAGE) and its variants in chronic obstructive pulmonary disease (COPD). In this study we have defined a role for RAGE in the pathogenesis of emphysema in mice. RAGE deficient mice (RAGE-/-) exposed to chronic cigarette smoke were significantly protected from smoke induced emphysema as determined by airspace enlargement and had no significant reduction in lung tissue elastance when compared to their air exposed controls contrary to their wild type littermates. The progression of emphysema has been largely attributed to an increased inflammatory cell-mediated elastolysis. Acute cigarette smoke exposure in RAGE-/- mice revealed an impaired early recruitment of neutrophils, approximately a 6-fold decrease compared to wild type mice. Hence, impaired neutrophil recruitment with continued cigarette smoke exposure reduces elastolysis and consequent emphysema.
Conflict of interest statement
Figures

Similar articles
-
Emphysema requires the receptor for advanced glycation end-products triggering on structural cells.Am J Respir Cell Mol Biol. 2015 Apr;52(4):482-91. doi: 10.1165/rcmb.2014-0027OC. Am J Respir Cell Mol Biol. 2015. PMID: 25188021
-
Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke.PLoS One. 2017 Jul 5;12(7):e0180092. doi: 10.1371/journal.pone.0180092. eCollection 2017. PLoS One. 2017. PMID: 28678851 Free PMC article.
-
Conditional overexpression of receptors for advanced glycation end-products in the adult murine lung causes airspace enlargement and induces inflammation.Am J Respir Cell Mol Biol. 2013 Jul;49(1):128-34. doi: 10.1165/rcmb.2013-0013OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526218
-
Role of receptor for advanced glycation end products (RAGE) in liver disease.Eur J Med Res. 2015 Feb 11;20(1):15. doi: 10.1186/s40001-015-0090-z. Eur J Med Res. 2015. PMID: 25888859 Free PMC article. Review.
-
Plausible Roles for RAGE in Conditions Exacerbated by Direct and Indirect (Secondhand) Smoke Exposure.Int J Mol Sci. 2017 Mar 17;18(3):652. doi: 10.3390/ijms18030652. Int J Mol Sci. 2017. PMID: 28304347 Free PMC article. Review.
Cited by
-
A Pilot Study Linking Endothelial Injury in Lungs and Kidneys in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2017 Jun 1;195(11):1464-1476. doi: 10.1164/rccm.201609-1765OC. Am J Respir Crit Care Med. 2017. PMID: 28085500 Free PMC article.
-
Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers.Antioxidants (Basel). 2023 Jun 3;12(6):1210. doi: 10.3390/antiox12061210. Antioxidants (Basel). 2023. PMID: 37371940 Free PMC article. Review.
-
Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling.FASEB J. 2017 May;31(5):2076-2089. doi: 10.1096/fj.201601155R. Epub 2017 Feb 1. FASEB J. 2017. PMID: 28148566 Free PMC article.
-
HHIP protein interactions in lung cells provide insight into COPD pathogenesis.Hum Mol Genet. 2025 Apr 17;34(9):777-789. doi: 10.1093/hmg/ddaf016. Hum Mol Genet. 2025. PMID: 39945347
-
Applying Functional Genomics to Chronic Obstructive Pulmonary Disease.Ann Am Thorac Soc. 2018 Dec;15(Suppl 4):S239-S242. doi: 10.1513/AnnalsATS.201808-530MG. Ann Am Thorac Soc. 2018. PMID: 30759001 Free PMC article. Review.
References
-
- Snider GL (1989) Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annual review of medicine 40: 411–429. - PubMed
-
- Shapiro SD, Snider GL, Rennard SI, Broadus VC, Murray JF, Nadel JA (eds) (2005) Textbook of respiratory medicine. Philadelphia: Elsevier.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

