Development of a single nucleotide polymorphism barcode to genotype Plasmodium vivax infections
- PMID: 25781890
- PMCID: PMC4362761
- DOI: 10.1371/journal.pntd.0003539
Development of a single nucleotide polymorphism barcode to genotype Plasmodium vivax infections
Abstract
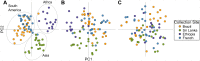
Plasmodium vivax, one of the five species of Plasmodium parasites that cause human malaria, is responsible for 25-40% of malaria cases worldwide. Malaria global elimination efforts will benefit from accurate and effective genotyping tools that will provide insight into the population genetics and diversity of this parasite. The recent sequencing of P. vivax isolates from South America, Africa, and Asia presents a new opportunity by uncovering thousands of novel single nucleotide polymorphisms (SNPs). Genotyping a selection of these SNPs provides a robust, low-cost method of identifying parasite infections through their unique genetic signature or barcode. Based on our experience in generating a SNP barcode for P. falciparum using High Resolution Melting (HRM), we have developed a similar tool for P. vivax. We selected globally polymorphic SNPs from available P. vivax genome sequence data that were located in putatively selectively neutral sites (i.e., intergenic, intronic, or 4-fold degenerate coding). From these candidate SNPs we defined a barcode consisting of 42 SNPs. We analyzed the performance of the 42-SNP barcode on 87 P. vivax clinical samples from parasite populations in South America (Brazil, French Guiana), Africa (Ethiopia) and Asia (Sri Lanka). We found that the P. vivax barcode is robust, as it requires only a small quantity of DNA (limit of detection 0.3 ng/μl) to yield reproducible genotype calls, and detects polymorphic genotypes with high sensitivity. The markers are informative across all clinical samples evaluated (average minor allele frequency > 0.1). Population genetic and statistical analyses show the barcode captures high degrees of population diversity and differentiates geographically distinct populations. Our 42-SNP barcode provides a robust, informative, and standardized genetic marker set that accurately identifies a genomic signature for P. vivax infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

