Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps
- PMID: 25781931
- PMCID: PMC4363326
- DOI: 10.1371/journal.pone.0118464
Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps
Abstract
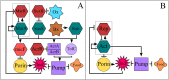
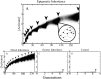
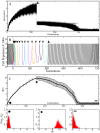
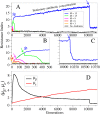
Adaptive resistance emerges when populations of bacteria are subjected to gradual increases of antibiotics. It is characterized by a rapid emergence of resistance and fast reversibility to the non-resistant phenotype when the antibiotic is removed from the medium. Recent work shows that adaptive resistance requires epigenetic inheritance and heterogeneity of gene expression patterns that are, in particular, associated with the production of porins and efflux pumps. However, the precise mechanisms by which inheritance and variability govern adaptive resistance, and what processes cause its reversibility remain unclear. Here, using an efflux pump regulatory network (EPRN) model, we show that the following three mechanisms are essential to obtain adaptive resistance in a bacterial population: 1) intrinsic variability in the expression of the EPRN transcription factors; 2) epigenetic inheritance of the transcription rate of EPRN associated genes; and 3) energetic cost of the efflux pumps activity that slows down cell growth. While the first two mechanisms acting together are responsible for the emergence and gradual increase of the resistance, the third one accounts for its reversibility. In contrast with the standard assumption, our model predicts that adaptive resistance cannot be explained by increased mutation rates. Our results identify the molecular mechanism of epigenetic inheritance as the main target for therapeutic treatments against the emergence of adaptive resistance. Finally, our theoretical framework unifies known and newly identified determinants such as the burden of efflux pumps that underlie bacterial adaptive resistance to antibiotics.
Conflict of interest statement
Figures
References
-
- Cohen SP, McMurry LM, Hooper DC, Wolfson JS, Levy SB. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction Antimicrob Agents Chemother 1989. May. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

