Assembly and interrogation of Alzheimer's disease genetic networks reveal novel regulators of progression
- PMID: 25781952
- PMCID: PMC4363671
- DOI: 10.1371/journal.pone.0120352
Assembly and interrogation of Alzheimer's disease genetic networks reveal novel regulators of progression
Abstract
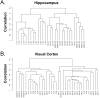
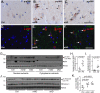
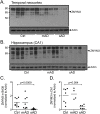
Alzheimer's disease (AD) is a complex multifactorial disorder with poorly characterized pathogenesis. Our understanding of this disease would thus benefit from an approach that addresses this complexity by elucidating the regulatory networks that are dysregulated in the neural compartment of AD patients, across distinct brain regions. Here, we use a Systems Biology (SB) approach, which has been highly successful in the dissection of cancer related phenotypes, to reverse engineer the transcriptional regulation layer of human neuronal cells and interrogate it to infer candidate Master Regulators (MRs) responsible for disease progression. Analysis of gene expression profiles from laser-captured neurons from AD and controls subjects, using the Algorithm for the Reconstruction of Accurate Cellular Networks (ARACNe), yielded an interactome consisting of 488,353 transcription-factor/target interactions. Interrogation of this interactome, using the Master Regulator INference algorithm (MARINa), identified an unbiased set of candidate MRs causally responsible for regulating the transcriptional signature of AD progression. Experimental assays in autopsy-derived human brain tissue showed that three of the top candidate MRs (YY1, p300 and ZMYM3) are indeed biochemically and histopathologically dysregulated in AD brains compared to controls. Our results additionally implicate p53 and loss of acetylation homeostasis in the neurodegenerative process. This study suggests that an integrative, SB approach can be applied to AD and other neurodegenerative diseases, and provide significant novel insight on the disease progression.
Conflict of interest statement
Figures
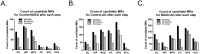


References
-
- WHO (2011) Global Health and Aging. World Health Organization.
-
- Selkoe DJ (1991) The molecular pathology of Alzheimer's disease. Neuron 6: 487–498. - PubMed
-
- Wilson CA, Doms RW, Lee VM (1999) Intracellular APP processing and A beta production in Alzheimer disease. J Neuropathol Exp Neurol 58: 787–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

