Crystal structure of Helicobacter pylori pseudaminic acid biosynthesis N-acetyltransferase PseH: implications for substrate specificity and catalysis
- PMID: 25781966
- PMCID: PMC4363471
- DOI: 10.1371/journal.pone.0115634
Crystal structure of Helicobacter pylori pseudaminic acid biosynthesis N-acetyltransferase PseH: implications for substrate specificity and catalysis
Abstract
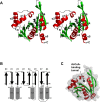
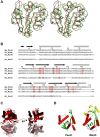
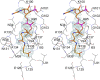
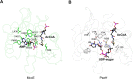
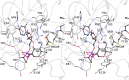
Helicobacter pylori infection is the common cause of gastroduodenal diseases linked to a higher risk of the development of gastric cancer. Persistent infection requires functional flagella that are heavily glycosylated with 5,7-diacetamido-3,5,7,9-tetradeoxy-L-glycero-L-manno-nonulosonic acid (pseudaminic acid). Pseudaminic acid biosynthesis protein H (PseH) catalyzes the third step in its biosynthetic pathway, producing UDP-2,4-diacetamido-2,4,6-trideoxy-β-L-altropyranose. It belongs to the GCN5-related N-acetyltransferase (GNAT) superfamily. The crystal structure of the PseH complex with cofactor acetyl-CoA has been determined at 2.3 Å resolution. This is the first crystal structure of the GNAT superfamily member with specificity to UDP-4-amino-4,6-dideoxy-β-L-AltNAc. PseH is a homodimer in the crystal, each subunit of which has a central twisted β-sheet flanked by five α-helices and is structurally homologous to those of other GNAT superfamily enzymes. Interestingly, PseH is more similar to the GNAT enzymes that utilize amino acid sulfamoyl adenosine or protein as a substrate than a different GNAT-superfamily bacterial nucleotide-sugar N-acetyltransferase of the known structure, WecD. Analysis of the complex of PseH with acetyl-CoA revealed the location of the cofactor-binding site between the splayed strands β4 and β5. The structure of PseH, together with the conservation of the active-site general acid among GNAT superfamily transferases, are consistent with a common catalytic mechanism for this enzyme that involves direct acetyl transfer from AcCoA without an acetylated enzyme intermediate. Based on structural homology with microcin C7 acetyltransferase MccE and WecD, the Michaelis complex can be modeled. The model suggests that the nucleotide- and 4-amino-4,6-dideoxy-β-L-AltNAc-binding pockets form extensive interactions with the substrate and are thus the most significant determinants of substrate specificity. A hydrophobic pocket accommodating the 6'-methyl group of the altrose dictates preference to the methyl over the hydroxyl group and thus to contributes to substrate specificity of PseH.
Conflict of interest statement
Figures
Similar articles
-
Structure and Functional Diversity of GCN5-Related N-Acetyltransferases (GNAT).Int J Mol Sci. 2016 Jun 28;17(7):1018. doi: 10.3390/ijms17071018. Int J Mol Sci. 2016. PMID: 27367672 Free PMC article. Review.
-
Cloning, purification and preliminary crystallographic analysis of the Helicobacter pylori pseudaminic acid biosynthesis N-acetyltransferase PseH.Acta Crystallogr F Struct Biol Commun. 2014 Sep;70(Pt 9):1276-9. doi: 10.1107/S2053230X14015398. Epub 2014 Aug 27. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25195909 Free PMC article.
-
Structural analysis of PseH, the Campylobacter jejuni N-acetyltransferase involved in bacterial O-linked glycosylation.Biochem Biophys Res Commun. 2015 Mar 20;458(4):843-8. doi: 10.1016/j.bbrc.2015.02.041. Epub 2015 Feb 16. Biochem Biophys Res Commun. 2015. PMID: 25698400
-
Structural and functional analysis of Campylobacter jejuni PseG: a udp-sugar hydrolase from the pseudaminic acid biosynthetic pathway.J Biol Chem. 2009 Jul 31;284(31):20989-1000. doi: 10.1074/jbc.M109.012351. Epub 2009 May 29. J Biol Chem. 2009. PMID: 19483088 Free PMC article.
-
The serine acetyltransferase reaction: acetyl transfer from an acylpantothenyl donor to an alcohol.Arch Biochem Biophys. 2005 Jan 1;433(1):85-95. doi: 10.1016/j.abb.2004.08.014. Arch Biochem Biophys. 2005. PMID: 15581568 Review.
Cited by
-
Bacterial GCN5-Related N-Acetyltransferases: From Resistance to Regulation.Biochemistry. 2016 Feb 23;55(7):989-1002. doi: 10.1021/acs.biochem.5b01269. Epub 2016 Feb 9. Biochemistry. 2016. PMID: 26818562 Free PMC article. Review.
-
Structure and Functional Diversity of GCN5-Related N-Acetyltransferases (GNAT).Int J Mol Sci. 2016 Jun 28;17(7):1018. doi: 10.3390/ijms17071018. Int J Mol Sci. 2016. PMID: 27367672 Free PMC article. Review.
-
The sps Genes Encode an Original Legionaminic Acid Pathway Required for Crust Assembly in Bacillus subtilis.mBio. 2020 Aug 18;11(4):e01153-20. doi: 10.1128/mBio.01153-20. mBio. 2020. PMID: 32817102 Free PMC article.
-
Post-translational Lysine Ac(et)ylation in Bacteria: A Biochemical, Structural, and Synthetic Biological Perspective.Front Microbiol. 2021 Oct 11;12:757179. doi: 10.3389/fmicb.2021.757179. eCollection 2021. Front Microbiol. 2021. PMID: 34721364 Free PMC article. Review.
-
Pathogenesis of Helicobacter pylori Infection.Helicobacter. 2015 Sep;20 Suppl 1(0 1):8-16. doi: 10.1111/hel.12251. Helicobacter. 2015. PMID: 26372819 Free PMC article. Review.
References
-
- Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186. - PubMed
-
- Bauer B, Meyer TF (2011) The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers Article ID 340157, 10.1155/2011/340157 - DOI
-
- Yoshiyama H, Nakazawa T (2000) Unique mechanism of Helicobacter pylori for colonizing the gastric mucus. Microbes Infect 2: 55–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

