The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review
- PMID: 25781999
- PMCID: PMC4363598
- DOI: 10.1371/journal.pone.0119683
The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review
Abstract
Background and objectives: Economic evaluations provide information to aid the optimal utilization of limited healthcare resources. Costs of biologics for Rheumatoid arthritis (RA) are remarkably high, which makes these agents an important target for economic evaluations. This systematic review aims to identify existing studies examining the cost-effectiveness of biologics for RA, assess their quality and report their results systematically.
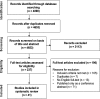
Methods: A literature search covering Medline, Scopus, Cochrane library, ACP Journal club and Web of Science was performed in March 2013. The cost-utility analyses (CUAs) of one or more available biological drugs for the treatment of RA in adults were included. Two independent investigators systematically collected information and assessed the quality of the studies. To enable the comparison of the results, all costs were converted to 2013 euro.
Results: Of the 4890 references found in the literature search, 41 CUAs were included in the current systematic review. While considering only direct costs, the incremental cost-effectiveness ratio (ICER) of the tumor necrosis factor inhibitors (TNFi) ranged from 39,000 to 1,273,000 €/quality adjusted life year (QALY) gained in comparison to conventional disease-modifying antirheumatic drugs (cDMARDs) in cDMARD naïve patients. Among patients with an insufficient response to cDMARDs, biologics were associated with ICERs ranging from 12,000 to 708,000 €/QALY. Rituximab was found to be the most cost-effective alternative compared to other biologics among the patients with an insufficient response to TNFi.
Conclusions: When 35,000 €/QALY is considered as a threshold for the ICER, TNFis do not seem to be cost-effective among cDMARD naïve patients and patients with an insufficient response to cDMARDs. With thresholds of 50,000 to 100,000 €/QALY biologics might be cost-effective among patients with an inadequate response to cDMARDs. Standardization of multiattribute utility instruments and a validated standard conversion method for missing utility measures would enable better comparison between CUAs.
Conflict of interest statement
Figures
References
-
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36: 182–188. - PubMed
-
- Lundkvist J, Kastäng F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ. 2008;8: S49–60. - PubMed
-
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73: 492–509. 10.1136/annrheumdis-2013-204573 - DOI - PMC - PubMed
-
- Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM a, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69: 976–986. 10.1136/ard.2009.126573 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical