Methyl-hydroxylamine as an efficacious antibacterial agent that targets the ribonucleotide reductase enzyme
- PMID: 25782003
- PMCID: PMC4363900
- DOI: 10.1371/journal.pone.0122049
Methyl-hydroxylamine as an efficacious antibacterial agent that targets the ribonucleotide reductase enzyme
Abstract
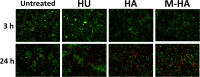
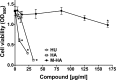
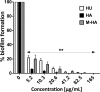
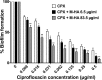
The emergence of multidrug-resistant bacteria has encouraged vigorous efforts to develop antimicrobial agents with new mechanisms of action. Ribonucleotide reductase (RNR) is a key enzyme in DNA replication that acts by converting ribonucleotides into the corresponding deoxyribonucleotides, which are the building blocks of DNA replication and repair. RNR has been extensively studied as an ideal target for DNA inhibition, and several drugs that are already available on the market are used for anticancer and antiviral activity. However, the high toxicity of these current drugs to eukaryotic cells does not permit their use as antibacterial agents. Here, we present a radical scavenger compound that inhibited bacterial RNR, and the compound's activity as an antibacterial agent together with its toxicity in eukaryotic cells were evaluated. First, the efficacy of N-methyl-hydroxylamine (M-HA) in inhibiting the growth of different Gram-positive and Gram-negative bacteria was demonstrated, and no effect on eukaryotic cells was observed. M-HA showed remarkable efficacy against Mycobacterium bovis BCG and Pseudomonas aeruginosa. Thus, given the M-HA activity against these two bacteria, our results showed that M-HA has intracellular antimycobacterial activity against BCG-infected macrophages, and it is efficacious in partially disassembling and inhibiting the further formation of P. aeruginosa biofilms. Furthermore, M-HA and ciprofloxacin showed a synergistic effect that caused a massive reduction in a P. aeruginosa biofilm. Overall, our results suggest the vast potential of M-HA as an antibacterial agent, which acts by specifically targeting a bacterial RNR enzyme.
Conflict of interest statement
Figures




Similar articles
-
Hydroxylamine Derivatives as a New Paradigm in the Search of Antibacterial Agents.ACS Omega. 2018 Dec 11;3(12):17057-17069. doi: 10.1021/acsomega.8b01384. eCollection 2018 Dec 31. ACS Omega. 2018. PMID: 31458325 Free PMC article.
-
Discovery of antimicrobial ribonucleotide reductase inhibitors by screening in microwell format.Proc Natl Acad Sci U S A. 2012 Jun 19;109(25):9798-803. doi: 10.1073/pnas.1113051109. Epub 2012 Jun 4. Proc Natl Acad Sci U S A. 2012. PMID: 22665797 Free PMC article.
-
Pseudomonas aeruginosa Nonphosphorylated AlgR Induces Ribonucleotide Reductase Expression under Oxidative Stress Infectious Conditions.mSystems. 2023 Apr 27;8(2):e0100522. doi: 10.1128/msystems.01005-22. Epub 2023 Feb 16. mSystems. 2023. PMID: 36794960 Free PMC article.
-
Ribonucleotide reductases: essential enzymes for bacterial life.Front Cell Infect Microbiol. 2014 Apr 28;4:52. doi: 10.3389/fcimb.2014.00052. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24809024 Free PMC article. Review.
-
Overview of ribonucleotide reductase inhibitors: an appealing target in anti-tumour therapy.Curr Med Chem. 2005;12(11):1283-94. doi: 10.2174/0929867054020981. Curr Med Chem. 2005. PMID: 15974997 Review.
Cited by
-
Profiling the compendium of changes in Saccharomyces cerevisiae due to mutations that alter availability of the main methyl donor S-Adenosylmethionine.G3 (Bethesda). 2024 Apr 3;14(4):jkae002. doi: 10.1093/g3journal/jkae002. G3 (Bethesda). 2024. PMID: 38184845 Free PMC article.
-
Hydroxylamine Derivatives as a New Paradigm in the Search of Antibacterial Agents.ACS Omega. 2018 Dec 11;3(12):17057-17069. doi: 10.1021/acsomega.8b01384. eCollection 2018 Dec 31. ACS Omega. 2018. PMID: 31458325 Free PMC article.
-
Design, Synthesis, and Antibacterial Activity of a Multivalent Polycationic Calix[4]arene-NO Photodonor Conjugate.ACS Med Chem Lett. 2017 Jul 5;8(8):881-885. doi: 10.1021/acsmedchemlett.7b00228. eCollection 2017 Aug 10. ACS Med Chem Lett. 2017. PMID: 28835806 Free PMC article.
-
Functionalized Calixarenes as Promising Antibacterial Drugs to Face Antimicrobial Resistance.Molecules. 2023 Oct 6;28(19):6954. doi: 10.3390/molecules28196954. Molecules. 2023. PMID: 37836797 Free PMC article. Review.
-
NO donors and NO delivery methods for controlling biofilms in chronic lung infections.Appl Microbiol Biotechnol. 2021 May;105(10):3931-3954. doi: 10.1007/s00253-021-11274-2. Epub 2021 May 3. Appl Microbiol Biotechnol. 2021. PMID: 33937932 Free PMC article. Review.
References
-
- Torrents E, Sahlin M, Sjöberg B-M. The Ribonucleotide Reductase Family- Genetics and Genomics In: Ribonucleotide Reductases (ed Andersson KK) Nova Science Publishers; 2008:pp. 17–77.
-
- Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

