Loxapine add-on for adolescents and adults with autism spectrum disorders and irritability
- PMID: 25782098
- PMCID: PMC4442591
- DOI: 10.1089/cap.2014.0003
Loxapine add-on for adolescents and adults with autism spectrum disorders and irritability
Abstract
Objectives: Our clinical experience with low dose loxapine (5-15 mg/day) suggests promising efficacy and safety for irritability in autism spectrum disorders (ASD). We studied low dose loxapine prospectively in adolescents and adults with ASD and irritability. Additionally, we measured loxapine and metabolite concentrations, and brain-derived neurotrophic factor (BDNF) as a biomarker of neuromodulation.
Methods: We performed a 12 week open trial of add-on loxapine in subjects, ages 13-65 years, diagnosed with ASD, and Aberrant Behavior Checklist-Irritability (ABC-I) subscale scores >14. Loxapine was dosed flexibly up to 15 mg daily, starting with 5 mg on alternate days. From weeks 1 to 6, other psychoactive medications were tapered if possible; from weeks 6 to 12, all medication doses were held stable. The primary outcome was the Clinical Global Impressions-Improvement subscale (CGI-I), ratings of Much Improved or Very Much Improved. Secondary outcomes were the ABC-I, Repetitive Behavior Scale-Revised, and Schalock Quality of Life scale. Serum BDNF and loxapine and metabolite concentrations were assayed. BDNF rs6265 was genotyped.
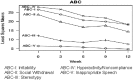
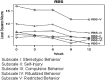
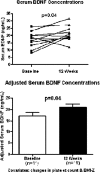
Results: Sixteen subjects were enrolled; 12 completed all visits. Median age was 18 years (range 13-39). Median final loxapine dose was 7.5 mg/day (2.5-15). All 14 subjects (100%) with data at week 12 were rated as Much Improved on CGI-I at 12 weeks. Mean change on ABC-I at 12 weeks was -31%, p=0.01. Mean body mass index (BMI)-Z decreased between weeks 6 and 12, p=0.03. Side effects were minimal, and prolactin elevation occurred in only one subject. BDNF concentrations measured in 11 subjects increased significantly (p=0.04). Subjects with AG genotype for BDNF rs6265 required a lower dose of loxapine at study end, but had similar behavioral and BDNF concentration changes as the GG genotype.
Conclusions: Low dose loxapine shows promise as a repurposed drug for irritability in ASD. Loxapine effects on BDNF warrant further study.
Figures


References
-
- Abdallah MW, Mortensen EL, Greaves-Lord K, Larsen N, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, Hougaard DM, Grove : Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr Scand 128:61–69, 2013 - PubMed
-
- Aman MG: Aberrant Behavior Checklist: Current identity and future developments. Clin Exp Pharmacol 2:e114, 2012
-
- Aman MG, Burrow WH, Wolford PL: The Aberrant Behavior Checklist-Community: Factor validity and effect of subject variables for adults in group homes. Am J Ment Retard 100:283–192, 1995 - PubMed
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

