Design, syntheses, and pharmacological characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3'-carboxamido)morphinan analogues as opioid receptor ligands
- PMID: 25783191
- PMCID: PMC4380750
- DOI: 10.1016/j.bmc.2015.02.055
Design, syntheses, and pharmacological characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3'-carboxamido)morphinan analogues as opioid receptor ligands
Abstract
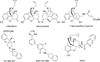
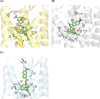
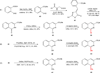
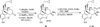
A series of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3'-carboxamido)morphinan (NAQ) analogues were synthesized and pharmacologically characterized to study their structure-activity relationship at the mu opioid receptor (MOR). The competition binding assay showed two-atom spacer and aromatic side chain were optimal for MOR selectivity. Meanwhile, substitutions at the 1'- and/or 4'-position of the isoquinoline ring retained or improved MOR selectivity over the kappa opioid receptor while still possessing above 20-fold MOR selectivity over the delta opioid receptor. In contrast, substitutions at the 6'- and/or 7'-position of the isoquinoline ring reduced MOR selectivity as well as MOR efficacy. Among this series of ligands, compound 11 acted as an antagonist when challenged with morphine in warm-water tail immersion assay and produced less significant withdrawal symptoms compared to naltrexone in morphine-pelleted mice. Compound 11 also antagonized the intracellular Ca(2+) increase induced by DAMGO. Molecular dynamics simulation studies of 11 in three opioid receptors indicated orientation of the 6'-nitro group varied significantly in the different 'address' domains of the receptors and played a crucial role in the observed binding affinities and selectivity. Collectively, the current findings provide valuable insights for future development of NAQ-based MOR selective ligands.
Keywords: Antagonist; MOR; SAR; Selectivity.
Published by Elsevier Ltd.
Figures
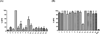
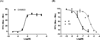
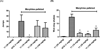

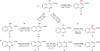

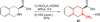
References
-
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. Pharmacol. Rev. 1996;48:567. - PubMed
-
- Chakrabarti S, Prather PL, Yu L, Law PY, Loh HH. J. Neurochem. 1995;64:2534. - PubMed
-
- Ortiz-Miranda SI, Dayanithi G, Coccia V, Custer EE, Alphandery S, Mazuc E, Treistman S, Lemos JR. J. Neuroendocrinol. 2003;15:888. - PubMed
-
- Barchfeld CC, Maasen ZF, Medzihradsky F. Life Sci. 1982;31:1661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

