In vivo chemical and structural analysis of plant cuticular waxes using stimulated Raman scattering microscopy
- PMID: 25783412
- PMCID: PMC4424026
- DOI: 10.1104/pp.15.00119
In vivo chemical and structural analysis of plant cuticular waxes using stimulated Raman scattering microscopy
Abstract
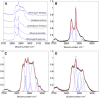
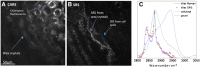
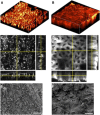
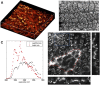
The cuticle is a ubiquitous, predominantly waxy layer on the aerial parts of higher plants that fulfils a number of essential physiological roles, including regulating evapotranspiration, light reflection, and heat tolerance, control of development, and providing an essential barrier between the organism and environmental agents such as chemicals or some pathogens. The structure and composition of the cuticle are closely associated but are typically investigated separately using a combination of structural imaging and biochemical analysis of extracted waxes. Recently, techniques that combine stain-free imaging and biochemical analysis, including Fourier transform infrared spectroscopy microscopy and coherent anti-Stokes Raman spectroscopy microscopy, have been used to investigate the cuticle, but the detection sensitivity is severely limited by the background signals from plant pigments. We present a new method for label-free, in vivo structural and biochemical analysis of plant cuticles based on stimulated Raman scattering (SRS) microscopy. As a proof of principle, we used SRS microscopy to analyze the cuticles from a variety of plants at different times in development. We demonstrate that the SRS virtually eliminates the background interference compared with coherent anti-Stokes Raman spectroscopy imaging and results in label-free, chemically specific confocal images of cuticle architecture with simultaneous characterization of cuticle composition. This innovative use of the SRS spectroscopy may find applications in agrochemical research and development or in studies of wax deposition during leaf development and, as such, represents an important step in the study of higher plant cuticles.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Baker EA, Holloway PJ (1971) Scanning electron microscopy of waxes on plant surfaces. Micron 2: 364–380
-
- Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202: 1–8
-
- Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (2008) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126: 237–260
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

