Attenuation of microglial RANTES by NEMO-binding domain peptide inhibits the infiltration of CD8(+) T cells in the nigra of hemiparkinsonian monkey
- PMID: 25783477
- PMCID: PMC4527882
- DOI: 10.1016/j.neuroscience.2015.03.011
Attenuation of microglial RANTES by NEMO-binding domain peptide inhibits the infiltration of CD8(+) T cells in the nigra of hemiparkinsonian monkey
Abstract
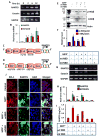
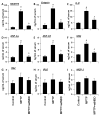
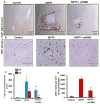
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Despite intense investigations, little is known about its pathological mediators. Here, we report the marked upregulation of RANTES (regulated on activation, normal T cell expressed and secreted) and eotaxin, chemokines that are involved in T cell trafficking, in the serum of hemiparkinsonian monkeys. Interestingly, 1-methyl-4-phenylpyridinium (MPP(+)), a Parkinsonian toxin, increased the expression of RANTES and eotaxin in mouse microglial cells. The presence of NF-κB binding sites in promoters of RANTES and eotaxin and down-regulation of these genes by NEMO-binding domain (NBD) peptide, selective inhibitor of induced NF-κB activation, in MPP(+)-stimulated microglial cells suggest that the activation of NF-κB plays an important role in the upregulation of these two chemokines. Consistently, serum enzyme-linked immuno assay (ELISA) and nigral immunohistochemistry further confirmed that these chemokines were strongly upregulated in MPTP-induced hemiparkinsonian monkeys and that treatment with NBD peptides effectively inhibited the level of these chemokines. Furthermore, the microglial upregulation of RANTES in the nigra of hemiparkinsonian monkeys could be involved in the altered adaptive immune response in the brain as we observed greater infiltration of CD8(+) T cells around the perivascular niche and deep brain parenchyma of hemiparkinsonian monkeys as compared to control. The treatment of hemiparkinsonian monkeys with NBD peptides decreased the microglial expression of RANTES and attenuated the infiltration of CD8(+) T cells in nigra. These results indicate the possible involvement of chemokine-dependent adaptive immune response in Parkinsonism.
Keywords: Parkinson’s disease; RANTES; T cell infiltration; hemiparkinsonian monkeys; microglia; neuroinflammation.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease.J Biol Chem. 2016 Jul 15;291(29):15267-81. doi: 10.1074/jbc.M116.714824. Epub 2016 May 12. J Biol Chem. 2016. Retraction in: J Biol Chem. 2025 Jan;301(1):108102. doi: 10.1016/j.jbc.2024.108102. PMID: 27226559 Free PMC article. Retracted.
-
RANTES-induced invasion of Th17 cells into substantia nigra potentiates dopaminergic cell loss in MPTP mouse model of Parkinson's disease.Neurobiol Dis. 2019 Dec;132:104575. doi: 10.1016/j.nbd.2019.104575. Epub 2019 Aug 22. Neurobiol Dis. 2019. PMID: 31445159 Free PMC article.
-
TNF-NF-kappaB signaling mediates excessive somnolence in hemiparkinsonian rats.Behav Brain Res. 2010 Apr 2;208(2):484-96. doi: 10.1016/j.bbr.2009.12.028. Epub 2010 Jan 4. Behav Brain Res. 2010. PMID: 20043954
-
Pathogenic role of glial cells in Parkinson's disease.Mov Disord. 2003 Feb;18(2):121-9. doi: 10.1002/mds.10332. Mov Disord. 2003. PMID: 12539204 Review.
-
Mechanisms of disease: regulation of RANTES (CCL5) in renal disease.Nat Clin Pract Nephrol. 2007 Mar;3(3):164-70. doi: 10.1038/ncpneph0418. Nat Clin Pract Nephrol. 2007. PMID: 17322928 Free PMC article. Review.
Cited by
-
Heparan sulfate-dependent phase separation of CCL5 and its chemotactic activity.Elife. 2024 Jul 1;13:RP93871. doi: 10.7554/eLife.93871. Elife. 2024. PMID: 38949655 Free PMC article.
-
Low-Dose Maraviroc, an Antiretroviral Drug, Attenuates the Infiltration of T Cells into the Central Nervous System and Protects the Nigrostriatum in Hemiparkinsonian Monkeys.J Immunol. 2019 Jun 15;202(12):3412-3422. doi: 10.4049/jimmunol.1800587. J Immunol. 2019. PMID: 31043478 Free PMC article.
-
Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury.J Immunol. 2016 Apr 15;196(8):3318-30. doi: 10.4049/jimmunol.1502021. Epub 2016 Mar 9. J Immunol. 2016. PMID: 26962232 Free PMC article.
-
T cells, α-synuclein and Parkinson disease.Handb Clin Neurol. 2022;184:439-455. doi: 10.1016/B978-0-12-819410-2.00023-0. Handb Clin Neurol. 2022. PMID: 35034753 Free PMC article. Review.
-
Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson's Disease.J Immunol. 2017 Jun 1;198(11):4312-4326. doi: 10.4049/jimmunol.1700149. Epub 2017 Apr 26. J Immunol. 2017. PMID: 28446566 Free PMC article.
References
-
- Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. - PubMed
-
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. - PMC - PubMed
-
- Czlonkowska A, Kurkowska-Jastrzebska I, Czlonkowski A, Peter D, Stefano GB. Immune processes in the pathogenesis of Parkinson’s disease - a potential role for microglia and nitric oxide. Med Sci Monit. 2002;8:RA165–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

