Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage
- PMID: 25783493
- PMCID: PMC4510924
- DOI: 10.1007/s00109-015-1275-1
Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage
Abstract
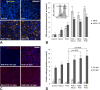
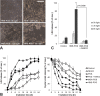
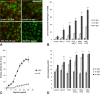
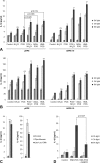
Photooxidative damage and chronic innate immune activation have been implicated in retinal pigment epithelium (RPE) dysfunction, a process that underlies blinding diseases such as age-related macular degeneration (AMD). To identify a potential molecular link between these mechanisms, we investigated whether lipofuscin-mediated phototoxicity activates the NLRP3 inflammasome in RPE cells in vitro. We found that blue light irradiation (dominant wavelength 448 nm, irradiance 0.8 mW/cm(2), duration 6 h) of lipofuscin-loaded primary human RPE cells and ARPE-19 cells induced photooxidative damage, lysosomal membrane permeabilization (79.5 % of cells vs. 3.8 % in nonirradiated controls), and cytosolic leakage of lysosomal enzymes. This resulted in activation of the inflammasome with activation of caspase-1 and secretion of interleukin-1β (14.6 vs. 0.9 pg/ml in nonirradiated controls) and interleukin-18 (87.7 vs. 0.2 pg/ml in nonirradiated controls). Interleukin secretion was dependent on the activity of NLRP3, caspase-1, and lysosomal proteases cathepsin B and L. These results demonstrate that accumulation of lipofuscin-like material in vitro renders RPE cells susceptible to phototoxic destabilization of lysosomes, resulting in NLRP3 inflammasome activation and secretion of inflammatory cytokines. This new mechanism of inflammasome activation links photooxidative damage and innate immune activation in RPE pathology and may provide novel targets for therapeutic intervention in retinal diseases such as AMD.
Key message: • Visible light irradiation of lipofuscin-loaded RPE cells activates inflammasome. • Inflammasome activation results from lysosomal permeabilization and enzyme leakage. • Inflammasome activation induces secretion of inflammatory cytokines by RPE cells. • Photooxidative damage by visible light as new mechanism of inflammasome activation. • Novel link between hallmark pathogenetic features of retinal degenerative diseases.
Figures


Similar articles
-
Complement Component C5a Primes Retinal Pigment Epithelial Cells for Inflammasome Activation by Lipofuscin-mediated Photooxidative Damage.J Biol Chem. 2015 Dec 25;290(52):31189-98. doi: 10.1074/jbc.M115.671180. Epub 2015 Nov 12. J Biol Chem. 2015. PMID: 26565031 Free PMC article.
-
NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration.Invest Ophthalmol Vis Sci. 2013 Jan 7;54(1):110-20. doi: 10.1167/iovs.12-10655. Invest Ophthalmol Vis Sci. 2013. PMID: 23221073 Free PMC article.
-
Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis.J Photochem Photobiol B. 2016 Aug;161:177-83. doi: 10.1016/j.jphotobiol.2016.05.018. Epub 2016 May 21. J Photochem Photobiol B. 2016. PMID: 27240191
-
Autophagy in dry AMD: A promising therapeutic strategy for retinal pigment epithelial cell damage.Exp Eye Res. 2024 May;242:109889. doi: 10.1016/j.exer.2024.109889. Epub 2024 Apr 7. Exp Eye Res. 2024. PMID: 38593971 Review.
-
Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration.Acta Ophthalmol. 2012 Jun;90(4):299-309. doi: 10.1111/j.1755-3768.2011.02179.x. Epub 2011 Nov 23. Acta Ophthalmol. 2012. PMID: 22112056 Review.
Cited by
-
Hypoxic expression of NLRP3 and VEGF in cultured retinal pigment epithelial cells: contribution of P2Y2 receptor signaling.Purinergic Signal. 2018 Dec;14(4):471-484. doi: 10.1007/s11302-018-9631-6. Epub 2018 Nov 10. Purinergic Signal. 2018. PMID: 30415294 Free PMC article.
-
Long-Chain Polyunsaturated Fatty Acids and Their Metabolites Regulate Inflammation in Age-Related Macular Degeneration.J Inflamm Res. 2022 Feb 9;15:865-880. doi: 10.2147/JIR.S347231. eCollection 2022. J Inflamm Res. 2022. PMID: 35173457 Free PMC article. Review.
-
Efficacy of novel selective NLRP3 inhibitors in human and murine retinal pigment epithelial cells.J Mol Med (Berl). 2019 Apr;97(4):523-532. doi: 10.1007/s00109-019-01753-5. Epub 2019 Feb 10. J Mol Med (Berl). 2019. PMID: 30739141
-
Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo.J Cell Mol Med. 2017 Dec;21(12):3453-3466. doi: 10.1111/jcmm.13255. Epub 2017 Jun 29. J Cell Mol Med. 2017. PMID: 28661040 Free PMC article.
-
IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases.Front Immunol. 2019 Jul 16;10:1618. doi: 10.3389/fimmu.2019.01618. eCollection 2019. Front Immunol. 2019. PMID: 31379825 Free PMC article. Review.
References
-
- Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

