Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1
- PMID: 25783564
- PMCID: PMC4511357
- DOI: 10.1111/jcmm.12534
Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1
Abstract
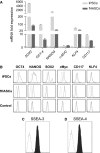
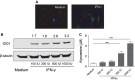
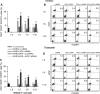
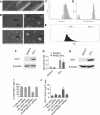
Although human amniotic fluid does contain different populations of foetal-derived stem cells, scanty information is available on the stemness and the potential immunomodulatory activity of in vitro expanded, amniotic fluid stem cells. By means of a methodology unrequiring immune selection, we isolated and characterized different stem cell types from second-trimester human amniotic fluid samples (human amniotic fluid stem cells, HASCs). Of those populations, one was characterized by a fast doubling time, and cells were thus designated as fHASCs. Cells maintained their original phenotype under prolonged in vitro passaging, and they were able to originate embryoid bodies. Moreover, fHASCs exhibited regulatory properties when treated with interferon (IFN)-γ, including induction of the immunomodulatory enzyme indoleamine 2,3-dioxygenase 1 (IDO1). On coculture with human peripheral blood mononuclear cells, IFN-γ-treated fHASCs caused significantly decreased T-cell proliferation and increased frequency in CD4(+) CD25(+) FOXP3(+) regulatory T cells. Both effects required an intact IDO1 function and were cell contact-independent. An unprecedented finding in our study was that purified vesicles from IFN-γ-treated fHASCs abundantly expressed the functional IDO1 protein, and those vesicles were endowed with an fHASC-like regulatory function. In vivo, fHASCs were capable of immunoregulatory function, promoting allograft survival in a mouse model of allogeneic skin transplantation. This was concurrent with the expansion of CD4(+) CD25(+) Foxp3(+) T cells in graft-draining lymph nodes from recipient mice. Thus fHASCs, or vesicles thereof, may represent a novel opportunity for immunoregulatory maneuvers both in vitro and in vivo.
Keywords: T cells; cell culture; cloning; immunosuppression; lymphocytes; pluripotent stem cells.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
S1P promotes migration, differentiation and immune regulatory activity in amniotic-fluid-derived stem cells.Eur J Pharmacol. 2018 Aug 15;833:173-182. doi: 10.1016/j.ejphar.2018.06.005. Epub 2018 Jun 7. Eur J Pharmacol. 2018. PMID: 29886240 Free PMC article.
-
Comparative proteomic analysis of two distinct stem-cell populations from human amniotic fluid.Mol Biosyst. 2015 Jun;11(6):1622-32. doi: 10.1039/c5mb00018a. Epub 2015 Mar 26. Mol Biosyst. 2015. PMID: 25811139
-
Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis.J Tissue Eng Regen Med. 2019 Oct;13(10):1792-1804. doi: 10.1002/term.2930. Epub 2019 Jul 25. J Tissue Eng Regen Med. 2019. PMID: 31293088
-
Interferon-γ converts human microvascular pericytes into negative regulators of alloimmunity through induction of indoleamine 2,3-dioxygenase 1.JCI Insight. 2018 Mar 8;3(5):e97881. doi: 10.1172/jci.insight.97881. JCI Insight. 2018. PMID: 29515027 Free PMC article.
-
Therapeutic potential of amniotic fluid stem cells.Curr Stem Cell Res Ther. 2013 Mar;8(2):117-24. doi: 10.2174/1574888x11308020002. Curr Stem Cell Res Ther. 2013. PMID: 23157178 Review.
Cited by
-
Body fluid-derived stem cells - an untapped stem cell source in genitourinary regeneration.Nat Rev Urol. 2023 Dec;20(12):739-761. doi: 10.1038/s41585-023-00787-2. Epub 2023 Jul 6. Nat Rev Urol. 2023. PMID: 37414959 Free PMC article. Review.
-
Amniotic fluid stem cell-derived extracellular vesicles educate type 2 conventional dendritic cells to rescue autoimmune disorders in a multiple sclerosis mouse model.J Extracell Vesicles. 2024 Jun;13(6):e12446. doi: 10.1002/jev2.12446. J Extracell Vesicles. 2024. PMID: 38844736 Free PMC article.
-
Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics.Int J Mol Sci. 2022 Jan 6;23(2):590. doi: 10.3390/ijms23020590. Int J Mol Sci. 2022. PMID: 35054775 Free PMC article. Review.
-
GBP1 Facilitates Indoleamine 2,3-Dioxygenase Extracellular Secretion to Promote the Malignant Progression of Lung Cancer.Front Immunol. 2021 Jan 20;11:622467. doi: 10.3389/fimmu.2020.622467. eCollection 2020. Front Immunol. 2021. PMID: 33552086 Free PMC article.
-
Human amniotic fluid as a source of stem cells.Open Med (Wars). 2022 Apr 4;17(1):648-660. doi: 10.1515/med-2022-0468. eCollection 2022. Open Med (Wars). 2022. PMID: 35434378 Free PMC article.
References
-
- Prusa A-R, Marton E, Rosner M, et al. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–93. - PubMed
-
- Priest RE, Marimuthu KM, Priest JH. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest. 1978;39:106–9. - PubMed
-
- Tsai M-S, Lee J-L, Chang Y-J, et al. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

