Ornithine-δ-Aminotransferase Inhibits Neurogenesis During Xenopus Embryonic Development
- PMID: 25783604
- PMCID: PMC4554259
- DOI: 10.1167/iovs.15-16509
Ornithine-δ-Aminotransferase Inhibits Neurogenesis During Xenopus Embryonic Development
Abstract
Purpose: In humans, deficiency of ornithine-δ-aminotransferase (OAT) results in progressive degeneration of the neural retina (gyrate atrophy) with blindness in the fourth decade. In this study, we used the Xenopus embryonic developmental model to study functions of the OAT gene on embryonic development.
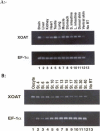
Methods: We cloned and sequenced full-length OAT cDNA from Xenopus oocytes (X-OAT) and determined X-OAT expression in various developmental stages of Xenopus embryos and in a variety of adult tissues. The phenotype, gene expression of neural developmental markers, and enzymatic activity were detected by gain-of-function and loss-of-function manipulations.
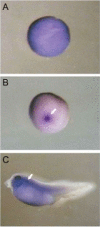
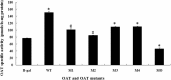
Results: We showed that X-OAT is essential for Xenopus embryonic development, and overexpression of X-OAT produces a ventralized phenotype characterized by a small head, lack of axial structure, and defective expression of neural developmental markers. Using X-OAT mutants based on mutations identified in humans, we found that substitution of both Arg 180 and Leu 402 abrogated both X-OAT enzymatic activity and ability to modulate the developmental phenotype. Neurogenesis is inhibited by X-OAT during Xenopus embryonic development.
Conclusions: Neurogenesis is inhibited by X-OAT during Xenopus embryonic development, but it is essential for Xenopus embryonic development. The Arg 180 and Leu 402 are crucial for these effects of the OAT molecule in development.
Figures







Similar articles
-
Expression of human ornithine aminotransferase (OAT) in OAT-deficient Chinese hamster ovary cells and fibroblasts of gyrate atrophy patient.Jpn J Ophthalmol. 1992;36(1):28-32. Jpn J Ophthalmol. 1992. PMID: 1635292
-
The ornithine aminotransferase gene in gyrate atrophy of the retina: analysis of expression and gross structure of this gene in cultured fibroblasts.In Vitro Cell Dev Biol. 1989 Oct;25(10):971-6. doi: 10.1007/BF02624012. In Vitro Cell Dev Biol. 1989. PMID: 2808228
-
Gyrate atrophy of the choroid and retina: lymphocyte ornithine-delta-aminotransferase activity in different mutations and carriers.Pediatr Res. 1998 Sep;44(3):381-5. doi: 10.1203/00006450-199809000-00019. Pediatr Res. 1998. PMID: 9727717
-
Calcium transients and calcium signalling during early neurogenesis in the amphibian embryo Xenopus laevis.Biochim Biophys Acta. 2006 Nov;1763(11):1184-91. doi: 10.1016/j.bbamcr.2006.08.005. Epub 2006 Aug 10. Biochim Biophys Acta. 2006. PMID: 16987559 Review.
-
Molecular pathology of gyrate atrophy of the choroid and retina due to ornithine aminotransferase deficiency.Mol Biol Med. 1991 Feb;8(1):81-93. Mol Biol Med. 1991. PMID: 1682785 Review.
Cited by
-
Ornithine aminotransferase and carbamoyl phosphate synthetase 1 involved in ammonia metabolism serve as novel targets for early stages of gastric cancer.J Clin Lab Anal. 2022 Oct;36(10):e24692. doi: 10.1002/jcla.24692. Epub 2022 Sep 13. J Clin Lab Anal. 2022. PMID: 36098904 Free PMC article.
-
Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways.Biology (Basel). 2017 Mar 7;6(1):18. doi: 10.3390/biology6010018. Biology (Basel). 2017. PMID: 28272331 Free PMC article. Review.
References
-
- Hagedorn CH,, Phang JM. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986; 248: 166–174. - PubMed
-
- Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 1985; 25: 91–132. - PubMed
-
- Fremeau RT, Jr,, Caron MG,, Blakely RD. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992; 8: 915–926. - PubMed
-
- Gogos JA,, Santha M,, Takacs Z,, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999; 21: 434–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

