Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation
- PMID: 25783969
- PMCID: PMC4575230
- DOI: 10.1038/mi.2015.16
Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation
Abstract
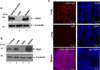
The epithelial lining of the airway tract and allergen-specific IgE are considered essential controllers of inflammatory responses to allergens. The human low affinity IgE receptor, CD23 (FcɛRII), is capable of transporting IgE or IgE-allergen complexes across the polarized human airway epithelial cell (AEC) monolayer in vitro. However, it remains unknown whether the CD23-dependent IgE transfer pathway in AECs initiates and facilitates allergic inflammation in vivo, and whether inhibition of this pathway attenuates allergic inflammation. To this end, we show that in wild-type (WT) mice, epithelial CD23 transcytosed both IgE and ovalbumin (OVA)-IgE complexes across the airway epithelial barrier, whereas neither type of transcytosis was observed in CD23 knockout (KO) mice. In chimeric mice, OVA sensitization and aerosol challenge of WT/WT (bone-marrow transfer from the WT to WT) or CD23KO/WT (CD23KO to WT) chimeric mice, which express CD23 on radioresistant airway structural cells (mainly epithelial cells) resulted in airway eosinophilia, including collagen deposition and a significant increase in goblet cells, and increased airway hyperreactivity. In contrast, the absence of CD23 expression on airway structural or epithelial cells, but not on hematopoietic cells, in WT/CD23KO (the WT to CD23KO) chimeric mice significantly reduced OVA-driven allergic airway inflammation. In addition, inhalation of the CD23-blocking B3B4 antibody in sensitized WT mice before or during airway challenge suppressed the salient features of asthma, including bronchial hyperreactivity. Taken together, these results identify a previously unproven mechanism in which epithelial CD23 plays a central role in the development of allergic inflammation. Further, our study suggests that functional inhibition of CD23 in the airway is a potential therapeutic approach to inhibit the development of asthma.
Figures


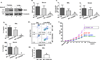


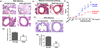
Similar articles
-
CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells.J Immunol. 2011 Mar 15;186(6):3484-96. doi: 10.4049/jimmunol.1002146. Epub 2011 Feb 9. J Immunol. 2011. PMID: 21307287
-
In vivo intranasal anti-CD23 treatment inhibits allergic responses in a murine model of allergic rhinitis.J Mol Histol. 2013 Jun;44(3):327-38. doi: 10.1007/s10735-013-9484-9. Epub 2013 Feb 3. J Mol Histol. 2013. PMID: 23377922
-
CD23 deficient mice develop allergic airway hyperresponsiveness following sensitization with ovalbumin.Am J Respir Crit Care Med. 1997 Dec;156(6):1945-55. doi: 10.1164/ajrccm.156.6.9701087. Am J Respir Crit Care Med. 1997. PMID: 9412579
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
IgE in allergy and asthma today.Nat Rev Immunol. 2008 Mar;8(3):205-17. doi: 10.1038/nri2273. Nat Rev Immunol. 2008. PMID: 18301424 Review.
Cited by
-
Membrane Transport across Polarized Epithelia.Cold Spring Harb Perspect Biol. 2017 Sep 1;9(9):a027912. doi: 10.1101/cshperspect.a027912. Cold Spring Harb Perspect Biol. 2017. PMID: 28213463 Free PMC article. Review.
-
The role of CD23 in the regulation of allergic responses.Allergy. 2021 Jul;76(7):1981-1989. doi: 10.1111/all.14724. Epub 2021 Jan 16. Allergy. 2021. PMID: 33378583 Free PMC article. Review.
-
The Direct and Indirect Role of IgE on Airway Epithelium in Asthma.Allergy. 2025 Apr;80(4):919-931. doi: 10.1111/all.16459. Epub 2025 Feb 18. Allergy. 2025. PMID: 39963805 Free PMC article. Review.
-
The rationale for development of ligelizumab in food allergy.World Allergy Organ J. 2022 Sep 13;15(9):100690. doi: 10.1016/j.waojou.2022.100690. eCollection 2022 Sep. World Allergy Organ J. 2022. PMID: 36185545 Free PMC article. Review.
-
Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy against chronic tuberculosis.Cell Mol Immunol. 2021 Oct;18(10):2393-2401. doi: 10.1038/s41423-020-0439-2. Epub 2020 May 7. Cell Mol Immunol. 2021. PMID: 32382128 Free PMC article.
References
-
- Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013;14:536–542. - PubMed
-
- Crimi E, Scordamaglia A, Crimi P, Zupo S, Barocci S. Total and specific IgE in serum, bronchial lavage and bronchoalveolar lavage of asthmatic patients. Allergy. 1983;38:553–559. - PubMed
-
- Fiset PO, Cameron L, Hamid Q. Local isotype switching to IgE in airway mucosa. J Allergy Clin Immunol. 2005;116:233–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

