IP-10 measured by Dry Plasma Spots as biomarker for therapy responses in Mycobacterium Tuberculosis infection
- PMID: 25783975
- PMCID: PMC4363864
- DOI: 10.1038/srep09223
IP-10 measured by Dry Plasma Spots as biomarker for therapy responses in Mycobacterium Tuberculosis infection
Abstract
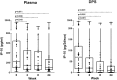
Tuberculosis (TB) has huge impact on human morbidity and mortality and biomarkers to support rapid TB diagnosis and ensure treatment initiation and cure are needed, especially in regions with high prevalence of multi-drug resistant TB. Soluble interferon gamma inducible protein 10 (IP-10) analyzed from dry plasma spots (DPS) has potential as an immunodiagnostic marker in TB infection. We analyzed IP-10 levels in plasma directly and extracted from DPS in parallel by ELISA from 34 clinically well characterized patients with TB disease before and throughout 24 weeks of effective anti-TB chemotherapy. We detected a significant decline of IP-10 levels in both plasma and DPS already after two weeks of therapy with good correlation between the tests. This was observed both in pulmonary and extrapulmonary TB. In conclusion, plasma IP-10 may serve as an early biomarker for anti-TB chemotherapy responses and the IP-10 DPS method has potential to be developed into a point-of care test for use in resource-limited settings. Further studies must be performed to validate the use of IP-10 DPS in TB high endemic countries.
Figures



Similar articles
-
IP-10 dried blood spots assay monitoring treatment efficacy in extrapulmonary tuberculosis in a low-resource setting.Sci Rep. 2019 Mar 7;9(1):3871. doi: 10.1038/s41598-019-40458-0. Sci Rep. 2019. PMID: 30846768 Free PMC article.
-
Dried plasma spots in the diagnosis of tuberculosis: IP-10 release assay on filter paper.Eur Respir J. 2013 Aug;42(2):495-503. doi: 10.1183/09031936.00129412. Epub 2013 Jan 24. Eur Respir J. 2013. PMID: 23349445 Free PMC article.
-
IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy.J Infect. 2015 Apr;70(4):381-91. doi: 10.1016/j.jinf.2014.12.019. Epub 2015 Jan 15. J Infect. 2015. PMID: 25597826
-
[CXCL10/IP-10 as a new biomarker for Mycobacterium tuberculosis infection].Pol Merkur Lekarski. 2012 Dec;33(198):342-5. Pol Merkur Lekarski. 2012. PMID: 23437705 Review. Polish.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
Cited by
-
Interplay of DDP4 and IP-10 as a Potential Mechanism for Cell Recruitment to Tuberculosis Lesions.Front Immunol. 2018 Jul 5;9:1456. doi: 10.3389/fimmu.2018.01456. eCollection 2018. Front Immunol. 2018. PMID: 30026741 Free PMC article.
-
High sensitivity and specificity of a 5-analyte protein and microRNA biosignature for identification of active tuberculosis.Clin Transl Immunology. 2021 Jun 22;10(6):e1298. doi: 10.1002/cti2.1298. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34188917 Free PMC article.
-
Combined Analysis of IFN-γ, IL-2, IL-5, IL-10, IL-1RA and MCP-1 in QFT Supernatant Is Useful for Distinguishing Active Tuberculosis from Latent Infection.PLoS One. 2016 Apr 1;11(4):e0152483. doi: 10.1371/journal.pone.0152483. eCollection 2016. PLoS One. 2016. PMID: 27035669 Free PMC article.
-
CXCL9/CXCL10 as biomarkers the monitoring of treatment responses in Pulmonary TB patients: a systematic review and meta-analysis.BMC Infect Dis. 2024 Sep 27;24(1):1037. doi: 10.1186/s12879-024-09939-0. BMC Infect Dis. 2024. PMID: 39333908 Free PMC article.
-
Diagnostic accuracy of interferon-gamma-induced protein 10 for differentiating active tuberculosis from latent tuberculosis: A meta-analysis.Sci Rep. 2019 Aug 6;9(1):11408. doi: 10.1038/s41598-019-47923-w. Sci Rep. 2019. PMID: 31388072 Free PMC article.
References
-
- World health organization. Global Tuberculosis report 2014. Available at: http://www.who.int/tb/publications/global_report/en/. (Accessed 12 December 2014).
-
- Wallis R. S. et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect. Dis. 13, 362–372 (2013). - PubMed
-
- Gandhi N. R. et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375, 1830–1843 (2010). - PubMed
-
- Heyckendorf J., Olaru I. D., Ruhwald M. & Lange C. Getting personal perspectives on individualized treatment duration in multidrug-resistant and extensively drug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 190, 374–383 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

