Mechanisms and Regulation of Alternative Pre-mRNA Splicing
- PMID: 25784052
- PMCID: PMC4526142
- DOI: 10.1146/annurev-biochem-060614-034316
Mechanisms and Regulation of Alternative Pre-mRNA Splicing
Abstract
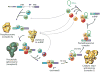
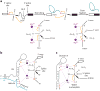
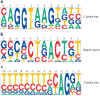
Precursor messenger RNA (pre-mRNA) splicing is a critical step in the posttranscriptional regulation of gene expression, providing significant expansion of the functional proteome of eukaryotic organisms with limited gene numbers. Split eukaryotic genes contain intervening sequences or introns disrupting protein-coding exons, and intron removal occurs by repeated assembly of a large and highly dynamic ribonucleoprotein complex termed the spliceosome, which is composed of five small nuclear ribonucleoprotein particles, U1, U2, U4/U6, and U5. Biochemical studies over the past 10 years have allowed the isolation as well as compositional, functional, and structural analysis of splicing complexes at distinct stages along the spliceosome cycle. The average human gene contains eight exons and seven introns, producing an average of three or more alternatively spliced mRNA isoforms. Recent high-throughput sequencing studies indicate that 100% of human genes produce at least two alternative mRNA isoforms. Mechanisms of alternative splicing include RNA-protein interactions of splicing factors with regulatory sites termed silencers or enhancers, RNA-RNA base-pairing interactions, or chromatin-based effects that can change or determine splicing patterns. Disease-causing mutations can often occur in splice sites near intron borders or in exonic or intronic RNA regulatory silencer or enhancer elements, as well as in genes that encode splicing factors. Together, these studies provide mechanistic insights into how spliceosome assembly, dynamics, and catalysis occur; how alternative splicing is regulated and evolves; and how splicing can be disrupted by cis- and trans-acting mutations leading to disease states. These findings make the spliceosome an attractive new target for small-molecule, antisense, and genome-editing therapeutic interventions.
Keywords: RNA structure; RNA-binding proteins; disease; enhancers; exon; genomics; intron; pre-mRNA splicing; silencers; spliceosome; splicing factors.
Figures
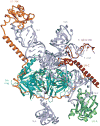


References
-
- Sharp PA. Split genes and RNA splicing. Cell. 1994;77:805–15. - PubMed
-
- Sharp PA. The discovery of split genes and RNA splicing. Trends Biochem Sci. 2005;30:279–81. - PubMed
-
- Kim H, Klein R, Majewski J, Ott J. Estimating rates of alternative splicing in mammals and invertebrates. Nat Genet. 2004;36:915–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

