The Clothes Make the mRNA: Past and Present Trends in mRNP Fashion
- PMID: 25784054
- PMCID: PMC4804868
- DOI: 10.1146/annurev-biochem-080111-092106
The Clothes Make the mRNA: Past and Present Trends in mRNP Fashion
Abstract
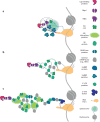
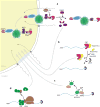
Throughout their lifetimes, messenger RNAs (mRNAs) associate with proteins to form ribonucleoproteins (mRNPs). Since the discovery of the first mRNP component more than 40 years ago, what is known as the mRNA interactome now comprises >1,000 proteins. These proteins bind mRNAs in myriad ways with varying affinities and stoichiometries, with many assembling onto nascent RNAs in a highly ordered process during transcription and precursor mRNA (pre-mRNA) processing. The nonrandom distribution of major mRNP proteins observed in transcriptome-wide studies leads us to propose that mRNPs are organized into three major domains loosely corresponding to 5' untranslated regions (UTRs), open reading frames, and 3' UTRs. Moving from the nucleus to the cytoplasm, mRNPs undergo extensive remodeling as they are first acted upon by the nuclear pore complex and then by the ribosome. When not being actively translated, cytoplasmic mRNPs can assemble into large multi-mRNP assemblies or be permanently disassembled and degraded. In this review, we aim to give the reader a thorough understanding of past and current eukaryotic mRNP research.
Keywords: RNA granules; RNA processing; RNP remodeling; coupling; mRNP packaging; ribonucleoproteins.
Figures


References
-
- Gall JG. On the submicroscopic structure of chromosomes. Brookhaven Symp Biol. 1956;8:17–32. - PubMed
-
- Dreyfuss G. Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu Rev Cell Biol. 1986;2:459–98. - PubMed
-
- Kumar A, Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975;96:353–65. - PubMed
-
- Beyer AL, Christensen ME, Walker BW, LeStourgeon WM. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–38. - PubMed
-
- Barrieux A, Ingraham HA, David DN, Rosenfeld MG. Isolation of messenger-like ribonucleoproteins. Biochemistry. 1975;14:1815–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

