Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis
- PMID: 25784679
- PMCID: PMC4440886
- DOI: 10.1182/blood-2014-10-608315
Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis
Abstract
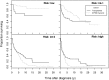
Allogeneic hematopoietic stem cell transplantation (SCT) is the only curative option for patients with primary myelofibrosis (PMF), but information on its net advantage over conventional therapies is lacking. Using ad hoc statistical analysis, we determined outcomes in 438 patients <65 years old at diagnosis who received allogenic SCT (n = 190) or conventional therapies (n = 248). Among patients at low risk per the Dynamic International Prognostic Scoring System (DIPSS) model, the relative risk of death after allogenic SCT vs those treated with nontransplant modalities was 5.6 (95% CI, 1.7-19; P = .0051); for intermediate-1 risk it was 1.6 (95% CI, 0.79-3.2; P = .19), for intermediate-2 risk, 0.55 (95% CI, 0.36-0.83; P = .005), and for high risk, 0.37 (95% CI, 0.21-0.66; P = .0007). Thus, patients with intermediate-2 or high-risk PMF clearly benefit from allogenic SCT. Patients at low risk should receive nontransplant therapy, whereas individual counseling is indicated for patients at intermediate-1 risk.
© 2015 by The American Society of Hematology.
Figures
References
-
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. - PubMed
-
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. - PubMed
-
- Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114(26):5264–5270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources