Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1
- PMID: 25784705
- PMCID: PMC4453520
- DOI: 10.1128/mBio.02486-14
Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1
Abstract
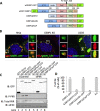
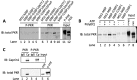
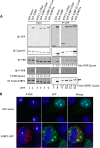
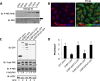
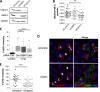
Stress granules (SGs) are dynamic cytoplasmic repositories containing translationally silenced mRNAs that assemble upon cellular stress. We recently reported that the SG nucleating protein G3BP1 promotes antiviral activity and is essential in double-stranded RNA-dependent protein kinase (PKR) recruitment to stress granules, thereby driving phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α). Here, we delineate the mechanism for SG-dependent PKR activation. We show that G3BP1 and inactive PKR directly interact with each other, dependent on both the NTF2-like and PXXP domains of G3BP1. The G3BP1-interacting protein Caprin1 also directly interacts with PKR, regulates efficient PKR activation at the stress granule, and is also integral for the release of active PKR into the cytoplasm to engage in substrate recognition. The G3BP1-Caprin1-PKR complex represents a new mode of PKR activation and is important for antiviral activity of G3BP1 and PKR during infection with mengovirus. Our data links stress responses and their resultant SGs with innate immune activation through PKR without a requirement for foreign double-stranded RNA (dsRNA) pattern recognition.
Importance: Our previous work indicates that stress granules have antiviral activity and mediate innate immunity through functions of G3BP1; however, the mechanistic details of these functions were not resolved. We show that much of the antiviral activity of stress granules is contingent on the function of PKR in a complex with G3BP1 and Caprin1. The PKR-G3BP1-Caprin1 complex undergoes dynamic transitioning within and outside stress granules to accomplish PKR activation and translational repression. This mechanism appears to function distinctly from canonical pattern recognition of double-stranded RNA by PKR. Therefore, this mechanism bridges the stress response with innate immunity, allowing the cell to respond to many cellular stressors and amplify the pathogen pattern recognition systems of innate immunity.
Copyright © 2015 Reineke et al.
Figures

Similar articles
-
The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses.J Virol. 2015 Mar;89(5):2575-89. doi: 10.1128/JVI.02791-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520508 Free PMC article.
-
Mouse Norovirus Infection Arrests Host Cell Translation Uncoupled from the Stress Granule-PKR-eIF2α Axis.mBio. 2019 Jun 18;10(3):e00960-19. doi: 10.1128/mBio.00960-19. mBio. 2019. PMID: 31213553 Free PMC article.
-
RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules.J Virol. 2020 Jun 16;94(13):e00205-20. doi: 10.1128/JVI.00205-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295917 Free PMC article.
-
A closer look at mammalian antiviral condensates.Biochem Soc Trans. 2024 Jun 26;52(3):1393-1404. doi: 10.1042/BST20231296. Biochem Soc Trans. 2024. PMID: 38778761 Free PMC article. Review.
-
Two Birds With One Stone: RNA Virus Strategies to Manipulate G3BP1 and Other Stress Granule Components.Wiley Interdiscip Rev RNA. 2025 Mar-Apr;16(2):e70005. doi: 10.1002/wrna.70005. Wiley Interdiscip Rev RNA. 2025. PMID: 40170442 Free PMC article. Review.
Cited by
-
A proposed role for the SARS-CoV-2 nucleocapsid protein in the formation and regulation of biomolecular condensates.FASEB J. 2020 Aug;34(8):9832-9842. doi: 10.1096/fj.202001351. Epub 2020 Jun 20. FASEB J. 2020. PMID: 32562316 Free PMC article.
-
Current Status of Epitranscriptomic Marks Affecting lncRNA Structures and Functions.Noncoding RNA. 2022 Mar 28;8(2):23. doi: 10.3390/ncrna8020023. Noncoding RNA. 2022. PMID: 35447886 Free PMC article. Review.
-
Norovirus infection results in eIF2α independent host translation shut-off and remodels the G3BP1 interactome evading stress granule formation.PLoS Pathog. 2020 Jan 6;16(1):e1008250. doi: 10.1371/journal.ppat.1008250. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31905230 Free PMC article.
-
Strategies for Success. Viral Infections and Membraneless Organelles.Front Cell Infect Microbiol. 2019 Oct 11;9:336. doi: 10.3389/fcimb.2019.00336. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31681621 Free PMC article. Review.
-
A little less aggregation a little more replication: Viral manipulation of stress granules.Wiley Interdiscip Rev RNA. 2023 Jan;14(1):e1741. doi: 10.1002/wrna.1741. Epub 2022 Jun 16. Wiley Interdiscip Rev RNA. 2023. PMID: 35709333 Free PMC article. Review.
References
-
- Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, Schrader JW. 2007. Distinct structural features of Caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol Cell Biol 27:2324–2342. doi:10.1128/MCB.02300-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA125123/CA/NCI NIH HHS/United States
- HD007495/HD/NICHD NIH HHS/United States
- R01 GM111700/GM/NIGMS NIH HHS/United States
- T32 HD007495/HD/NICHD NIH HHS/United States
- R56 AI050237/AI/NIAID NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01 AI050237/AI/NIAID NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- CA168872/CA/NCI NIH HHS/United States
- DK56338/DK/NIDDK NIH HHS/United States
- AI50237/AI/NIAID NIH HHS/United States
- R01 CA168872/CA/NCI NIH HHS/United States
- GM111700-01/GM/NIGMS NIH HHS/United States
- P30 HD007495/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

