Poly(ethylene glycol)-block-poly(ε-caprolactone)-and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery
- PMID: 25784805
- PMCID: PMC4356685
- DOI: 10.2147/IJN.S75186
Poly(ethylene glycol)-block-poly(ε-caprolactone)-and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery
Abstract
Background: Effective anticancer drug delivery to the tumor site without rapid body clearance is a prerequisite for successful chemotherapy. 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-(methoxy[polyethyleneglycol]-2000) (DSPE-PEG2000) has been widely used in the preparation of stealth liposomes. Although PEG chains can efficiently preserve liposomes from rapid clearance by the reticuloendothelial system (RES), its application has been hindered by poor cellular uptake and unsatisfactory therapeutic effect.
Methods: To address the dilemma, we presented a facile approach to fabricate novel stealth nanoparticles generated by poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL), soybean phosphatidylcholine (SPC), and cholesterol, namely LPPs (L represented lipid and PP represented PEG-b-PCL), for the delivery of anticancer drug paclitaxel (PTX). LPPs were prepared using the thin film hydration method. Two PEG-b-PCL polymers with different molecular weights (MW; PEG2000-b-PCL2000, MW: 4,000 Da and PEG5000-b-PCL5000, MW: 10,000 Da) were used to fabricate stealth nanoparticles. Conventional PEGylated liposome (LDP2000, L represented lipid and DP2000 represented DSPE-PEG2000) composed of SPC, cholesterol, and DSPE-PEG2000 was used as the control. The physical properties, cellular uptake, endocytosis pathway, cytotoxicity, pharmacokinetics, tumor accumulation, and anticancer efficacy of free PTX, PTX-loaded LPPs, and LDP2000 were systemically investigated after injection into 4T1 breast tumor-bearing mice.
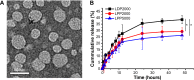
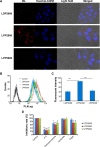
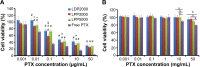
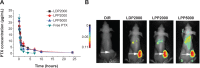
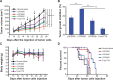
Results: LPPs were vesicles around 100 nm in size with negative zeta potential. With enhanced stability, LPPs achieved sustainable release of cancer therapeutics. The cellular uptake level was closely related to the PEG chain length of PEG-b-PCL; a shorter PEG chain resulted in higher cellular uptake. Moreover, the cellular internalization of LPP2000 modified by PEG2000-b-PCL2000 on 4T1 cells was 2.1-fold higher than LDP2000 due to the improved stability of LPP2000. The cytotoxicity of PTX-loaded LPP2000 was also higher than that of LDP2000 and LPP5000 as observed using a WST-8 assay, while blank LPPs showed negligible toxicity. Consistent with the results of the in vitro study, in vivo experiments showed that LPPs allowed significantly improved bioavailability and prolonged T1/2β as compared to free PTX injection. More importantly, LPPs mainly accumulated at the tumor site, probably due to the enhanced permeation and retention effect (EPR effect). As a nanomedicine, LPP2000 (tumor inhibition rate of 75.1%) significantly enhanced the therapeutic effect of PTX in 4T1 breast tumor-bearing mice by inhibiting tumor growth compared to LDP2000 and LPP5000 (tumor inhibition rates of 56.3% and 49.5%, respectively).
Conclusion: Modification of liposomes with PEG2000-b-PCL2000 can simultaneously improve drug accumulation at the target tumor site and tumor cells, showing great promise for utilization as a PEG modification tool in the fabrication of stealth nanoparticles for cancer chemotherapy.
Keywords: murine breast cancer chemotherapy; nanoparticles PEG-b-PCL; paclitaxel; phospholipid.
Figures
Similar articles
-
Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery.Int J Nanomedicine. 2015 Mar 16;10:2101-14. doi: 10.2147/IJN.S77667. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25844039 Free PMC article.
-
Synthesis and evaluation of a paclitaxel-binding polymeric micelle for efficient breast cancer therapy.Sci China Life Sci. 2018 Apr;61(4):436-447. doi: 10.1007/s11427-017-9274-9. Epub 2018 Mar 19. Sci China Life Sci. 2018. PMID: 29572777
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Paclitaxel-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles for lung cancer therapy.Int J Nanomedicine. 2013;8:1947-57. doi: 10.2147/IJN.S44220. Epub 2013 May 16. Int J Nanomedicine. 2013. PMID: 23696703 Free PMC article.
-
Small interfering RNA for cancer treatment: overcoming hurdles in delivery.Acta Pharm Sin B. 2020 Nov;10(11):2075-2109. doi: 10.1016/j.apsb.2020.10.005. Epub 2020 Oct 13. Acta Pharm Sin B. 2020. PMID: 33304780 Free PMC article. Review.
Cited by
-
Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come.Pharmacol Rev. 2016 Jul;68(3):701-87. doi: 10.1124/pr.115.012070. Pharmacol Rev. 2016. PMID: 27363439 Free PMC article. Review.
-
Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19-9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer.Theranostics. 2016 Jun 18;6(10):1573-87. doi: 10.7150/thno.15164. eCollection 2016. Theranostics. 2016. PMID: 27446491 Free PMC article.
-
SPIONs Conjugate Supported Anticancer Drug Doxorubicin's Delivery: Current Status, Challenges, and Prospects.Nanomaterials (Basel). 2022 Oct 20;12(20):3686. doi: 10.3390/nano12203686. Nanomaterials (Basel). 2022. PMID: 36296877 Free PMC article. Review.
-
Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses.Int J Mol Sci. 2016 Aug 31;17(9):1440. doi: 10.3390/ijms17091440. Int J Mol Sci. 2016. PMID: 27589733 Free PMC article. Review.
-
Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy.Int J Mol Sci. 2022 Dec 30;24(1):627. doi: 10.3390/ijms24010627. Int J Mol Sci. 2022. PMID: 36614071 Free PMC article.
References
-
- Slingerland M, Guchelaar HJ, Gelderblom H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov Today. 2012;17(3–4):160–166. - PubMed
-
- Forrest ML, Zhao A, Won CY, Malick AW, Kwon GS. Lipophilic prodrugs of Hsp90 inhibitor geldanamycin for nanoencapsulation in poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;116(2):139–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

