Clinical practice in febrile neutropenia risk assessment and granulocyte colony-stimulating factor primary prophylaxis of febrile neutropenia in Poland
- PMID: 25784841
- PMCID: PMC4355659
- DOI: 10.5114/wo.2014.47904
Clinical practice in febrile neutropenia risk assessment and granulocyte colony-stimulating factor primary prophylaxis of febrile neutropenia in Poland
Abstract
Aim of the study: The first aim was to investigate the knowledge and awareness of oncologists concerning febrile neutropenia (FN) risk assessment and indications for granulocyte colony-stimulating factor (G-CSF) primary prophylaxis (PP), based on current therapeutic guidelines (PTOK and EORTC). The second aim was to educate the oncologists on best practices for risk assessment and neutropenia management.
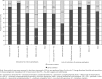
Material and methods: The project participants included 169 oncologists from 7 regions working in large specialist oncological centres, university hospitals, regional and city hospitals, specialist outpatient clinics, and oncological wards in small local hospitals. The participants completed a questionnaire based on seven prepared clinical cases of patients with different tumour types and patient characteristics, receiving chemotherapy (CT), and with different levels of FN risk. Participants answered questions related to FN risk assessment and G-CSF use. After completing the questionnaire, the participants proceeded to an educational module in which they were provided with an analysis of correct diagnostic and therapeutic procedures according to the PTOK and EORTC guidelines.
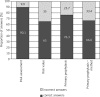
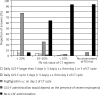
Results and conclusions: Febrile neutropenia risk assessment was found to be a routine procedure performed for over 90% of the clinical cases by the participant oncologists. However, the FN risk assessment of clinical cases was correct and consistent with therapeutic guidelines in only 65% of responses. Indications for G-CSF PP were properly identified in 76% of responses and it appeared that indications for G-CSF PP were more likely to be correctly identified in patients receiving high-risk or low-risk regimens than in those receiving intermediate-risk regimens, where the decision to give G-CSF PP is based on additional assessment of patient risk factors. The vast majority of participants who correctly identified the need for PP administered G-CSF in accordance with the dose and schedule recommended by PTOK and EORTC.
Keywords: G-CSF; chemotherapy induced neutropenia; febrile neutropenia prophylaxis.
Figures

References
-
- Potemski P. Neutropenia. In: Potemski P, Krzakowski M, editors. Zalecenia postępowania diagnostyczno-terapeutycznego w nowotworach złośliwych. Warszawa: PTOK; 2013. pp. 537–547.
-
- Aapro MS, Bohlius J, Cameron DA, et al. European Organisation for Research and Treatment of Cancer 2010 update of EORTC guidelines for the use of granulocyte- colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. - PubMed
-
- Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010;21(Suppl 5):v248–51. - PubMed
-
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. - PubMed
-
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–66. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
