Development of the choroid plexus and blood-CSF barrier
- PMID: 25784848
- PMCID: PMC4347429
- DOI: 10.3389/fnins.2015.00032
Development of the choroid plexus and blood-CSF barrier
Abstract
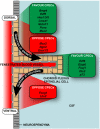
Well-known as one of the main sources of cerebrospinal fluid (CSF), the choroid plexuses have been, and still remain, a relatively understudied tissue in neuroscience. The choroid plexus and CSF (along with the blood-brain barrier proper) are recognized to provide a robust protective effort for the brain: a physical barrier to impede entrance of toxic metabolites to the brain; a "biochemical" barrier that facilitates removal of moieties that circumvent this physical barrier; and buoyant physical protection by CSF itself. In addition, the choroid plexus-CSF system has been shown to be integral for normal brain development, central nervous system (CNS) homeostasis, and repair after disease and trauma. It has been suggested to provide a stem-cell like repository for neuronal and astrocyte glial cell progenitors. By far, the most widely recognized choroid plexus role is as the site of the blood-CSF barrier, controller of the internal CNS microenvironment. Mechanisms involved combine structural diffusion restraint from tight junctions between plexus epithelial cells (physical barrier) and specific exchange mechanisms across the interface (enzymatic barrier). The current hypothesis states that early in development this interface is functional and more specific than in the adult, with differences historically termed as "immaturity" actually correctly reflecting developmental specialization. The advanced knowledge of the choroid plexus-CSF system proves itself imperative to understand a range of neurological diseases, from those caused by plexus or CSF drainage dysfunction (e.g., hydrocephalus) to more complicated late-stage diseases (e.g., Alzheimer's) and failure of CNS regeneration. This review will focus on choroid plexus development, outlining how early specializations may be exploited clinically.
Keywords: blood-CSF barrier; brain patterning; cerebrospinal fluid; choroid plexus; development; epithelia; neuroependyma.
Figures



References
-
- al-Sarraf H., Preston J. E., Segal M. B. (1997b). Changes in the kinetics of the acidic amino acid brain and CSF uptake during development in the rat. Brain Res. Dev. Brain Res. 102, 27–134. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

